Novel Device Preserves Donated Lungs For Cystic Fibrosis, Lung Disease Patients In Need of Transplant
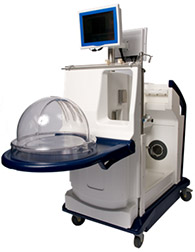
 The Food and Drug Administration (FDA) has given a Humanitarian Use Device approval to a novel device, the XPS™ and STEEN Solution™ XVIVO Perfusion System, which has the capacity to preserve donated lungs outside the human body for up to 4 hours before surgery into critically ill patients, including those suffering from cystic fibrosis (CF) who are anticipating a lung transplant
The Food and Drug Administration (FDA) has given a Humanitarian Use Device approval to a novel device, the XPS™ and STEEN Solution™ XVIVO Perfusion System, which has the capacity to preserve donated lungs outside the human body for up to 4 hours before surgery into critically ill patients, including those suffering from cystic fibrosis (CF) who are anticipating a lung transplant
Lung transplantation has been available for patients with CF for almost 30 years. The most common operation preformed is the double lung transplant, or Bilateral Sequential Lung Transplant, and over time, post-surgery survival rates have been constantly increasing, with about 85% of patients surviving for at least a year following the operation.
Even though lung transplantation can offer a new hope of life, there are still major risks to be considered. Besides the inherent surgical risks, there are still other factors to take into account, such as compatibility and preservation state, since only 1 in 5 lungs donated match the medical criteria for transplantation.
XVIVO Perfusion System is fabricated by Englewood, Colorado-based XVIVO Perfusion Inc., and consists of a bubble-like chamber that can store the donated lungs artificially. The organs are connected to pumps and filters that provide oxygen to the tissues and a sterile cleansing solution is added to preserve the lungs, which can be kept in the machine for 4 hours. This gives doctors enough time to examine the lungs, increasing the probabilities of a successful transplantation.
[adrotate group=”1″]
The company first submitted an FDA approval application in 2009, and throughout this time several clinical studies were preformed to demonstrate product and patient safety.
“It is a breakthrough for XVIVO that we have now received approval from the FDA and can initiate sales of STEEN Solution™ and XPS™ in the American market after a time-consuming and comprehensive process with high patient and product safety requirements. The XPS™ machine and STEEN Solution™ mean that XVIVO now has a clinically proven method that is both CE marked and approved by the FDA. This method allows more lungs to be used for transplantation, which will potentially enable more patients with severe lung disease to achieve a greater quality of life as well as a longer life,” said Dr. Magnus Nilsson, CEO of XVIVO Perfusion Inc., in the company’s press release.







