Could a Bilateral Lung and Pancreatic Transplantation From the Same Donor Benefit CFRD Patients?
 A team led by researchers at the University Hospital in Strasbourg, France is currently recruiting patients for a clinical trial designed to assess the efficacy of combined lung and pancreatic islet transplantation in individuals with end-stage cystic fibrosis (CF). The trial is entitled “Metabolic Efficiency of Combined Pancreatic Islet and Lung Transplant for the Treatment of End-Stage Cystic Fibrosis: a Pilot Study” (NCT01548729).
A team led by researchers at the University Hospital in Strasbourg, France is currently recruiting patients for a clinical trial designed to assess the efficacy of combined lung and pancreatic islet transplantation in individuals with end-stage cystic fibrosis (CF). The trial is entitled “Metabolic Efficiency of Combined Pancreatic Islet and Lung Transplant for the Treatment of End-Stage Cystic Fibrosis: a Pilot Study” (NCT01548729).
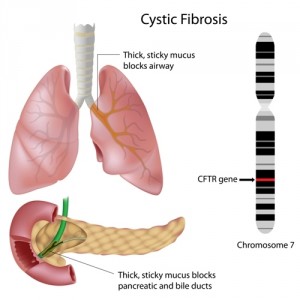
CF is a life-threatening genetic disease in which a defective gene causes the body to form unusually thick mucus that can result in serious respiratory and gastrointestinal manifestations. Individuals with CF often develop CF-related diabetes (CFRD), a unique type of diabetes that results from the fact that CF patients do not produce enough insulin or have insulin resistance (the body does not use insulin normally).
Bilateral lung and pancreatic islet transplantation has been shown to be a viable therapeutic option that can benefit patients with end-stage CF and severe CFRD. The primary goal of this Phase 1 and Phase 2 clinical trial is to assess the efficacy of the medical procedure in the treatment of CF and CFRD. Each patient will receive both organs from the same donor to reduce immunogenicity. The research team expects that the combined transplant may restore the metabolic control in the patient’s body, decreasing the complication rate in the postoperative period and improving the management of the disease.
[adrotate group=”1″]
End-stage CF patients who may benefit from a lung transplant, aged 18 to 60 years, of both genders, on insulin therapy and with CFRD duration longer than 3 years, no residual insulin secretion (C-peptide < 0.5 ng/mL) or no response to stimulation by intravenous glucagon (hormone that increases blood glucose) are eligible for the trial. The primary outcome measure will be a successful increase in C peptide for levels higher than 2 ng/mL in the follow-up period of 12 months after the transplant.
The research team is currently recruiting participants to enroll on this trial. For more information, please contact Dr. Laurence Kessler at 03 88 11 62 67 or send an e-mail to [email protected]. Several hospitals in France are involved in the recruiting process, for a list of the study locations please follow the link.







