KaloBios Continues KB001-A Trial For Pseudomonas Aeruginosa Infection in Cystic Fibrosis, Despite Dissolution of Sanofi Licensing Deal
Written by |

 San Francisco based KaloBios Pharmaceuticals, Inc. on Monday released an update on the status of its KB001-A monoclonal antibody development program, including news that multinational Sanofi Pasteur, which had been collaborating with Kalobios on the KB001-A R&D program, is pulling out of the partnership.
San Francisco based KaloBios Pharmaceuticals, Inc. on Monday released an update on the status of its KB001-A monoclonal antibody development program, including news that multinational Sanofi Pasteur, which had been collaborating with Kalobios on the KB001-A R&D program, is pulling out of the partnership.
KaloBios will regain all rights to KB001-A, and the collaboration and the 2010 licensing agreement with Sanofi Pasteur has been terminated. Under that agreement, Sanofi Pasteur had been developing KB001-A, a patented monoclonal antibody targeting Pseudomonas aeruginosa (Pa), for Pa pneumonia prevention in the intensive care setting, while KaloBios had been developing KB001-A for chronic treatment of Pa lung infections in cystic fibrosis (CF) patients.
“We are grateful to Sanofi for the work they did to advance the KB001-A program, including their global epidemiology study that supports KaloBios’ assumptions about the large market potential for KB001-A in ventilator-associated pneumonia (VAP). At the same time, we are pleased to regain the global rights for KB001-A across all indications,” commented David Pritchard, KaloBios’ President and Chief Executive Officer in a release. “We continue to believe in the potential of this innovative therapy as a means to address Pa infections in a variety of settings. This negotiated termination not only provides us with full unencumbered rights to our cystic fibrosis indication, but will enable us to seek a partner with established capabilities in additional indications as well as in territories outside of the United States. We will immediately embark on a process to identify a partner with a focus on infectious disease, hospital pharmaceuticals, or cystic fibrosis who can accelerate and financially support the pivotal studies for KB001-A. ”
Sanofi Pasteur has agreed to terminate the collaboration and licensing agreement with KaloBios in consideration of low single digit royalties on net sales of KB001-A, subject to a $40 million cap on the aggregate royalties to be paid. As Mr. Pritchard noted, KaloBios is now free to seek other potential corporate partners in development of the drug. Sanofi Pasteur will be entitled to receive up to 10% of certain sub-license payments or other milestone payments received in the event KaloBios successfully re-partners KB001-A, subject to a separate $40 million cap on the aggregate amount of sub-license payments to be shared with Sanofi Pasteur.
According to a report by FierceBiotech’s Damian Garde, KaloBios’ shares, already hammered by clinical troubles, dropped as much as 22 percent to about $1.50 on Monday, contributing to a swoon of 75 percent from their $8 debut in 2013. Garde says Sanofi paid $35 million to partner with KaloBios and had promised up to $255 million in further milestone funding.
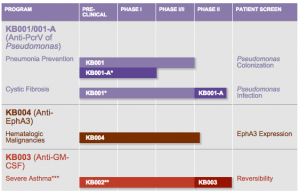 KB001-A is one of three KaloBios next generation customized monoclonal antibody agents being investigated in clinical development programs, and focused on treating serious respiratory diseases and cancer. The drugs are designed to overcome challenges limiting currently available marketed antibody products and engineered to enhance their desired effect and/or reduce possible undesired effects.
KB001-A is one of three KaloBios next generation customized monoclonal antibody agents being investigated in clinical development programs, and focused on treating serious respiratory diseases and cancer. The drugs are designed to overcome challenges limiting currently available marketed antibody products and engineered to enhance their desired effect and/or reduce possible undesired effects.
* KB001 precursor for KB001-A. Future studies with KB001-A.
** KB002 precursor for KB003. Phase 2 and future studies with KB003. KaloBios is currently evaluating other possible indications in order to determine next step, if any, in the development of KB003.
*** Based on the results of the Phase 2 study in severe asthma patients, KalyBios has discontinued development of KB003 in severe asthma.
KB001-A specifically targets prevention and treatment of Pseudomonas aeruginosa (Pa) infection. KaloBios has completed three clinical trials with an earlier functionally comparable molecule, KB001, in healthy human volunteers, cystic fibrosis patients infected with Pa, and Pa-colonized, mechanically ventilated patients hospitalized in the ICU. The company says results of these studies support further development in both indications.
KaloBios Moving Forward With KB001-A Clinical Trial for Cystic Fibrosis
KaloBios also announced that it has achieved full enrollment in a 180 patient Phase 2 study evaluating KB001-A in CF subjects with chronic Pa lung infection.
The purpose of this 180-patient Phase 2 multi-dose, randomized, double-blind, placebo-controlled clinical trial with KB001-A in cystic fibrosis patients infected with Pa. is to confirm and extend the Phase 1-2 KB001 findings of an airway anti-inflammatory effect in CF individuals with chronic Pseudomonas aeruginosa (Pa) airway infection. Researchers will evaluate the efficacy and safety of repeat doses of KB001-A. The primary endpoint is time-to-need for antibiotics.
It is hypothesized that steady-state levels of KB001-A in CF subjects with airway Pa infection will be safe and well-tolerated, and will increase the time-to-need for antibiotic treatment (IV, inhaled, or oral) for worsening of respiratory tract signs and symptoms compared with placebo.
“Enrollment increased dramatically in the last few months due to a number of factors, including additional ex-U.S. sites coming on line and enrolling patients, decreased competition from other studies in the cystic fibrosis space, and some seasonal benefit due to patient availability in the summer months,” explains Nestor A. Molfino, MD, MSc, Chief Medical Officer of KaloBios. “As a result we were able to make up ground and complete enrollment in the KB001-A Phase 2 study in a timeframe that should enable us to release top-line data in early first quarter 2015.”
KaloBios had previously completed a Phase ½ study with KB001 in thirty-five patients colonized with Pa, which demonstrated approximately a 50% reduction in VAP in patients treated with KB001. The study also showed a dose-dependent increase in bacterial event-free survival relative to placebo. The results of this study were published in Critical Care Medicine, and represented the primary efficacy data included in the Sanofi Pasteur regulatory submission under which the VAP indication received Fast Track Status from the FDA. In a separate Phase 1/2 study conducted with CF patients, KaloBios showed a trend towards a dose-dependent reduction in several key inflammatory markers, including neutrophil elastase, when measured 28 days after a single dose of KB001. The results of this study were published in Pediatric Pulmonology.
[adrotate group=”1″]
KaloBios has received Orphan Drug designation from both the U.S. FDA and the European Medicines Agency for KB001-A for the treatment of Pa lung infection in CF patients. KB001-A has also received Fast Track Status from the U.S. FDA for the prevention of ventilator associated pneumonia. KaloBios is planning to seek a partner to help accelerate the development of this program.
Orphan drug designation is granted by the FDA Office of Orphan Products Development to novel drugs or biologics that treat a rare disease or condition affecting fewer than 200,000 patients in the United States. The designation provides the drug developer with a seven-year period of U.S. marketing exclusivity if the drug is the first of its type approved for the specified indication or if it demonstrates superior safety, efficacy, or a major contribution to patient care versus another drug of its type that was previously granted the designation for the same indication. Orphan designation also provides tax credits for clinical research costs, the ability to apply for annual grant funding, clinical research trial design assistance and waiver of Prescription Drug User Fee Act (PDUFA) filing fees.
Pseudomonas aeruginosa and Cystic Fibrosis
The usually hospital-acquired bacterial pathogen Pseudomonas aeruginosa is particularly dangerous to persons with Cystic Fibrosis (CF), and reportedly is responsible for roughly 80 percent of pneumonia-induced deaths in CF patients, so KB001-A, if proven effective, could be a major breakthrough.
According to the Pseudomonas Genome Database, Pseudomonas aeruginosa is a Gram-negative bacterium noted for its environmental versatility, ability to cause disease in particular susceptible individuals, and its resistance to antibiotics. The Database notes that Pseudomonas aeruginosa respiratory tract infection is the most serious complication of Cystic Fibrosis, and that cancer and burn patients also commonly suffer serious infections by this organism, as do certain other individuals with immune systems deficiencies.
The Database explains that unlike many environmental bacteria, P. aeruginosa has a remarkable capacity to cause disease in susceptible hosts, and to adapt to and thrive in many ecological niches, from water and soil to plant and animal tissues. The bacterium is capable of utilizing a wide range of organic compounds as food sources, thus giving it an exceptional ability to colonize ecological niches where nutrients are limited. P. aeruginosa can also produce toxic proteins which not only cause extensive tissue damage, but also interfere with the human immune system’s defense mechanisms. These proteins range from toxins that enter and kill host cells at or near the site of colonization to degradative enzymes that permanently disrupt the cell membranes and connective tissues in various organs.
Among other things, Pseudomonas aeruginosa can cause inflammation of the skin and lungs in patients with a weak immune system or a chronic illness, and this bacterium is also noted for its resistance to many antibiotics. P. aeruginosa is widely studied by scientists who are interested in not only its ability to cause disease and resist antibiotics, but also its metabolic capability and environmental versatility. Analysis of its genome sequence has identified genes involved in locomotion, attachment, transport and utilization of nutrients, antibiotic efflux, and systems involved in sensing and responding to environmental changes.
More information about Pseudomonas aeruginosa, can be found at the Pseudomonas Genome Database frequently asked questions page.
Other KaloBios Developments
The other two monoclonal antibody agents in development by KaloBios are KB004 to treat patients with hematologic malignancies, and KB003 (anti-GM-CSF) to treat patients with severe asthma, and is currently being evaluated for other possible indications in order to determine next steps, if any, in the development of KB003.
KB004 is an anti-EphA3 mAb with potential in treating hematologic malignancies and solid tumors. KaloBios is running an ongoing Phase 1/2 study evaluating KB004 in hematologic malignancies. The Phase 1 dose escalation portion of that study in subjects with hematologic malignancies is ongoing. KaloBios initiated the Phase 2 expansion portion of the study focused on patients with certain EphA3 positive hematologic malignancies in early 2014.
KB003 is an anti-GM-CSF mAb with potential to treat inflammatory diseases that was being developed for the treatment of severe asthma. In early 2014, KaloBios completed a Phase 2 clinical study in 160 patients with severe asthma which did not meet its primary endpoint of improvement in FEV1 from baseline as compared to placebo. As a result, KaloBios discontinued development of this compound in severe asthma, and is continuing to analyze the Phase 2 data to review with thought leaders. KaloBios is currently evaluating other possible indications in order to determine next steps, if any, in the development of KB003.
All of the company’s antibodies are generated using its proprietary “Humaneered” technology, a method that converts nonhuman antibodies (typically mouse) into recombinant antibodies that have a high binding affinity to their target and are designed for chronic therapeutic use. The company believes that antibodies produced using its Humaneered technology offer important clinical and economic advantages over antibodies generated by other methods in terms of high binding affinity, high manufacturing yields, and minimal to no immunogenicity (inappropriate immune response) upon repeat administration in humans.
KaloBios uses a patient-targeted approach utilizing or developing a screen or diagnostic method to identify those patients most likely to benefit from our treatments. Ultimately, the company says this approach could result in better treatments than current therapies.
KaloBios Management hosted a teleconference and webcast to discuss plans for KB001-A in light of the updates on the KB001-A program on July 28, 2014. Individuals may access the live audio webcast by visiting the event URL at:
https://ir.kalobios.com/events.cfm
A replay of the webcast will be available on the Company’s website for six months following the live event.
Sources:
KaloBios Pharmaceuticals, Inc.
Pseudomonas Genome Database
FierceBiotech






