FAQs about types of CFTR mutations
Some treatments for cystic fibrosis (CF) will only work in patients with specific disease-causing mutations in the CFTR gene. Most notably, a class of medications called CFTR modulators can work to improve the functionality of the CFTR protein in people with specific disease-causing mutations, but these therapies will not work for people with other mutation types.
According to the Johns Hopkins Cystic Fibrosis Center, more than 2,500 different mutations in the CFTR gene have been documented.
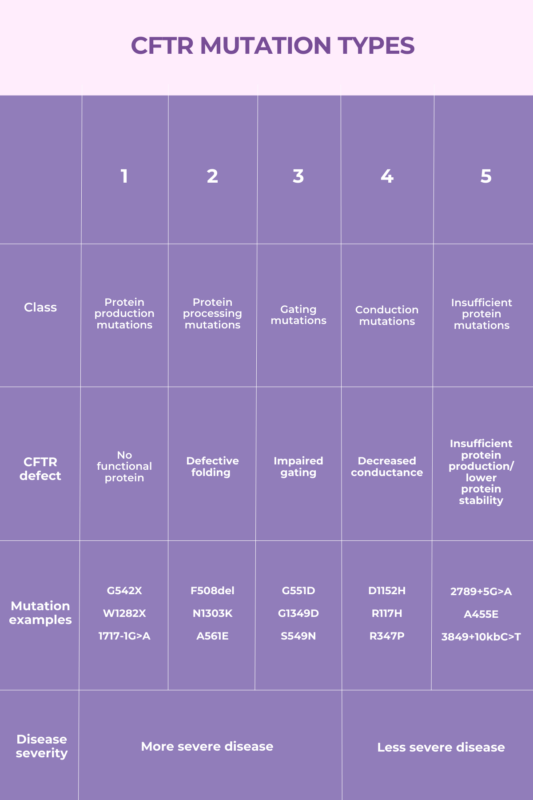
Mutations in the CFTR gene are classified based on their effect on the CFTR protein. A common modern classification system groups mutations into five classes: protein production mutations (class 1), protein processing mutations (class 2), gating mutations (class 3), conduction mutations (class 4), and insufficient protein mutations (class 5).
Mutations in the CFTR gene cause cystic fibrosis (CF). The CFTR gene provides instructions for making the CFTR protein, which is important for regulating the production of mucus in the body. Mutations in CFTR lead to the production of abnormally thick and sticky mucus which builds up in the body’s tissues, causing damage that drives most CF symptoms.
The most common mutation that causes cystic fibrosis (CF) is called F508del. This mutation is present in up to about 90% of CF patients, though it is more common in white populations than in other racial and ethnic groups.
Related Articles

 Fact-checked by
Fact-checked by