Researchers Find Sugar Agent Protects Cells Against CF Superbug Threat Pseudomonas Aeruginosa
Written by |
A team of research scientists at the University of Freiburg (Albert-Ludwigs-Universität Freiburg) at Freiburg im Breisgau, Baden-Württemberg, Germany, the University of Geneva, and the University of Grenoble report that they have succeeded in preventing the hospital-acquired bacteria Pseudomonas aeruginosa, a pathogen particularly dangerous to persons with Cystic Fibrosis (CF), from entering host cells with the help of a sugar complex. The bacterium is responsible for roughly 80 percent of pneumonia-induced deaths in CF patients, so this could be a major breakthrough discovery.
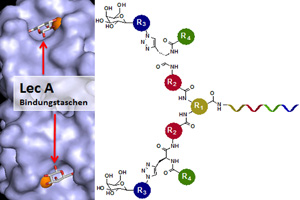
 European researchers have developed a molecule that prevents LecA from docking onto the host cell receptors by binding to the bacterial protein with great precision. (Image credit: Nicolas Winssinger, modified by BIOSS)
European researchers have developed a molecule that prevents LecA from docking onto the host cell receptors by binding to the bacterial protein with great precision. (Image credit: Nicolas Winssinger, modified by BIOSS)
Dr. Thorsten Eierhoff and junior professor Dr. Winfried Römer from the Institute of Biologie II, both members of the University of Freiburg Cluster of Excellence BIOSS Centre for Biological Signalling Studies, have identified a sugar complex that binds the bacterial protein LecA in close cooperation with research groups led by Prof. Dr. Nicolas Winssinger from the University of Geneva, Switzerland, and Dr. Anne Imberty from the University of Grenoble, France. This protein enables the “hospital germ” bacterium Pseudomonas aeruginosa to invade human lung cells, but in tests conducted in cell culture, the researchers demonstrated that the germs penetrate into human lung cells 90 percent less often when treated with the sugar-based agent.
The researchers published their findings in the weekly peer-reviewed scientific journal Angewandte Chemie. The report, coauthored by Dr. Alexandre Novoa, Dr. Thorsten Eierhoff, Dr. Jérémie Topin, Dr. Annabelle Varrot, Dr. Sofia Barluenga, Dr. Anne Imberty, Prof. Winfried Römer and Prof. Nicolas Winssinger, is entitled “A LecA Ligand Identified from a Galactoside-Conjugate Array Inhibits Host Cell Invasion by Pseudomonas aeruginosa”e (2014 – Angewandte Chemie: DOI: 10.1002/ange.201402831).
Pseudomonas aeruginosa
According to the Pseudomonas Genome Database, Pseudomonas aeruginosa is a Gram-negative bacterium noted for its environmental versatility, ability to cause disease in particular susceptible individuals, and its resistance to antibiotics. The Database notes that Pseudomonas aeruginosa respiratory tract infection is the most serious complication of Cystic Fibrosis, and that cancer and burn patients also commonly suffer serious infections by this organism, as do certain other individuals with immune systems deficiencies.
[adrotate group=”1″]
The Database explains that unlike many environmental bacteria, P. aeruginosa has a remarkable capacity to cause disease in susceptible hosts, and to adapt to and thrive in many ecological niches, from water and soil to plant and animal tissues. The bacterium is capable of utilizing a wide range of organic compounds as food sources, thus giving it an exceptional ability to colonize ecological niches where nutrients are limited. P. aeruginosa can produce a number of toxic proteins which not only cause extensive tissue damage, but also interfere with the human immune system’s defense mechanisms. These proteins range from potent toxins that enter and kill host cells at or near the site of colonization to degradative enzymes that permanently disrupt the cell membranes and connective tissues in various organs.
Among other things, Pseudomonas aeruginosa can cause inflammation of the skin and lungs in patients with a weak immune system or a chronic illness, and this bacterium is also noted for its resistance to many antibiotics. P. aeruginosa is widely studied by scientists who are interested in not only its ability to cause disease and resist antibiotics, but also its metabolic capability and environmental versatility. Analysis of its genome sequence has identified genes involved in locomotion, attachment, transport and utilization of nutrients, antibiotic efflux, and systems involved in sensing and responding to environmental changes. A major interest of pharmaceutical companies, such as Chiron (PathoGenesis Corporation, which played a major role in sequencing the first P. aeruginosa genome, is now a part of Chiron) is to learn more about the genes of P. aeruginosa and other disease-causing bacteria in order to better understand the physiology of these organisms. These insights will be used to develop new antibacterial drugs to successfully treat infections by bacteria like P. aeruginosa that are resistant to many of today’s antibiotics.
The University of Freiburg researchers report that the bacterial protein LecA binds the sugar galactose, which is exposed by receptors at the host cell surface. Via LecA, the bacterium attaches itself to the host cell and forces its way in. This allows the bacterium to spread through the body. The researchers developed a molecule that prevents LecA from docking onto the host cell receptors by binding to the bacterial protein with great precision. The research group led by Dr. Anne Imberty succeeded in illustrating how the interaction between the sugar and the LecA protein looks like at the molecular level. “The agent is tailored precisely to bind to LecA: A framework of organic molecules places two galactose sugars at the right distance from one another for them to fit snugly into two of LecA’s binding sites on the bacterial cell,” explains Dr. Eierhoff. The agent resembles a plug made of galactose sugars that fits perfectly into the socket of LecA. Once bound to LecA, the agent works like a protective cap, preventing the Pseudomonas bacteria from invading the host cell.
The release notes that the precisely fitting molecular framework was found by the research group led by the chemist Prof. Dr. Nicolas Winssinger of the University of Geneva. The team arranged molecules that were potential candidates for binding partners on a chip. This allowed the scientists to test many samples at once. They determined which plug fit best with the protein LecA. The group led by Römer then tested the best-fitting molecule in cell culture to measure how many bacteria still managed to invade cells under the influence of the plug.
The scientists explain that binding to host cells via LecA is a significant path of invasion for Pseudomonas aeruginosa in human lung cells, and hope their findings will lead to the development of a novel agent capable of substantially weakening the germ.
More information about Pseudomonas aeruginosa, can be found at the Pseudomonas Genome Database frequently asked questions page.
Albert-Ludwigs-Universität Freiburg was founded in 1457 by the Habsburg dynasty and is the fifth-oldest university in Germany, made up of 11 faculties with students from across Germany as over 120 other countries. Foreign students constitute about 16% of total student numbers. The University of Freiburg is one of Europe’s most prestigious universities and is amongst its top research and teaching institutions.
Sources:
Albert-Ludwigs-Universität Freiburg (University of Freiburg)
BIOSS Centre for Biological Signalling Studies
Pseudomonas Genome Database
Angewandte Chemie






