Cystic Fibrosis Patients Respond Well to Unique Antibacterial Ceragenins
Written by |
 Two recently published reviews in peer-reviewed journals plus another study highlight the potential utility of ceragenins or cationic selective antimicrobials (CSAs) — a proprietary, patented, first-in-class small molecule technology platform for fighting bacterial infections, such as pulmonary infections associated with cystic fibrosis (CF) and hospital acquired infections associated with medical devices.
Two recently published reviews in peer-reviewed journals plus another study highlight the potential utility of ceragenins or cationic selective antimicrobials (CSAs) — a proprietary, patented, first-in-class small molecule technology platform for fighting bacterial infections, such as pulmonary infections associated with cystic fibrosis (CF) and hospital acquired infections associated with medical devices.
Caragenin’s developer, Columbus, Ohio, based N8 Medical, is an emerging pharmaceutical company focused on addressing life-threatening global health challenges through development of high-value pharmaceutical and antimicrobial device solutions.
N8 says one of the lead ceragenin compounds, CSA-13, has exhibited particularly potent antimicrobial properties and is rapidly bactericidal, fungicidal and virucidal against a wide array of pathogens, including multidrug resistant strains of Methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (Pseudomonas). CSA-13 has also demonstrated capacity to both prevent and remove bacterial and fungal biofilms, which are nearly impossible to eradicate utilizing conventional antibiotics. They note that preclinical testing has displayed CSA-13’s high potency at low doses, and absence of toxic response at doses much higher than are expected to be clinically necessary.
The first review, “Design and synthesis of membrane-targeting antibiotics: from peptides- to aminosugar-based antimicrobial cationic amphiphiles” (pp. 1014–1026. Med. Chem. Commun., 2014, 5, 1014) published in the Royal Society of Medicine’s journal MedChemComm written by Drs. Ido Herzog and Micha Fridman, indicates that N8 Medical’s lead compound, CSA-13 may “…offer a promising opportunity for the development of clinically useful and novel antibiotic agents that are likely to be safe for …. the prevention of biofilm formation on medical equipment and devices….”
[adrotate group=”1″]
The second research paper, published in the journal Studia Medyczne, “Ceragenins a new weapon to fight multidrug resistant bacterial infections,” is coauthored by Urszula Surel, Katarzyna Niemirowicz, and Robert Bucki of the Department of Microbiological and Nanobiomedical Engineering, Medical University of Bialystok, Bialystok, Poland; Michal Marzec of the Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA; and Paul B. Savage of the Department of Chemistry and Biochemistry at Brigham Young University, Provo, UT.
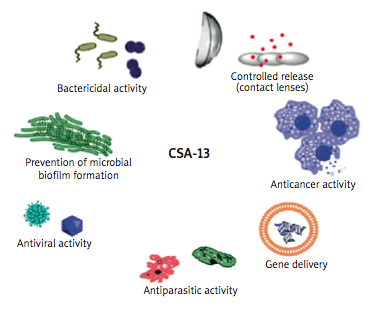 The researchers note that with antibiotic resistance among pathogenic microorganisms becoming one of the most challenging problems in health care, with even a single mutation in a bacterial cell often leading to formation of a new drug resistance mechanism, ceragenins represent a novel class of antibiotic, offering great promise in future treatment of infections. Their review focuses on ceragenins’ broad spectrum of antibacterial activity and their potential to become a new group of antibiotics for prevention and treatment of infections, especially those caused by multidrug-resistant bacteria.
The researchers note that with antibiotic resistance among pathogenic microorganisms becoming one of the most challenging problems in health care, with even a single mutation in a bacterial cell often leading to formation of a new drug resistance mechanism, ceragenins represent a novel class of antibiotic, offering great promise in future treatment of infections. Their review focuses on ceragenins’ broad spectrum of antibacterial activity and their potential to become a new group of antibiotics for prevention and treatment of infections, especially those caused by multidrug-resistant bacteria.
The review highlights the range of bacterial strains against which ceragenins may be useful, as well as providing an overview of the drug’s utility in fighting opportunistic infections afflicting persons with Cystic Fibrosis. The coauthors discuss various potential uses for CSA-13, which include prevention of microbial biofilm formation, as well as suggesting that CSA-13 may also have antiparasitic, antiviral, and anticancer applications and utility as a bactericide, as well as potential for employment in controlled release mechanisms (e.g., via contact lenses) and gene delivery.
 “Review articles such as these continue to validate our clinical hypotheses, encouraging our march to the clinic,” says N8 Medical’s CEO Dr. Michael Triplett. “Further, they demonstrate the multiple applications for ceragenins, which may ultimately provide N8 Medical with multiple opportunities for fighting bacterial infections, both in the near term as coatings for medical devices and in the longer term as potential therapeutics.”
“Review articles such as these continue to validate our clinical hypotheses, encouraging our march to the clinic,” says N8 Medical’s CEO Dr. Michael Triplett. “Further, they demonstrate the multiple applications for ceragenins, which may ultimately provide N8 Medical with multiple opportunities for fighting bacterial infections, both in the near term as coatings for medical devices and in the longer term as potential therapeutics.”
By leveraging the power and diversity of the ceragenin platform, N8 Medical says it has the potential to create significant value for patients, providers, and payers across several therapeutic categories and medical device segments. N8 Medical’s initial focus is to leverage the ceragenin technology’s antimicrobial activity and biofilm activity to address life-threatening infections associated with Cystic Fibrosis and airway management devices.
N8 Medical demonstrated CSA-13’s effectiveness against Cystic Fibrosis-associated lung infections at the 28th Annual North American Cystic Fibrosis Conference, held in Atlanta, GA in October, showing its efficacy as an antimicrobial peptide against Pseudomonas aeruginosa, a common persistent pulmonary infection associated with CF.
The study presented at the conference showed that the minimum inhibitory concentration (MIC) of CSA-13 (1.6-3.2 g/ml) was unchanged in the presence of sputum from CF patients, while the MIC of tobramycin (the antibiotic most commonly prescribes for pulmonary P. aeruginosa infections) increased significantly (from 0.8 g/ml to >64 mg/ml) in the presence of sputum from CF patients. CSA-13s MIC also remained unchanged whether tested in a tobramycin susceptible, or a tobramycin resistant strain (AGR 2).
 “This is an encouraging study, as it demonstrates the ability of CSA-13 to remain effective even in the presence of sputum from a cystic fibrosis patient, says Paul B. Savage, MD, Department of Chemistry and Biochemistry, Brigham Young University, who is credited with inventing ceragenins. “Further, it confirms previous findings that the compound may continue to be effective even in antibiotic-resistant strains of P. aeruginosa. While antibiotic resistance continues to be a concern, we are seeing potential, at least in this context, to find solutions.”
“This is an encouraging study, as it demonstrates the ability of CSA-13 to remain effective even in the presence of sputum from a cystic fibrosis patient, says Paul B. Savage, MD, Department of Chemistry and Biochemistry, Brigham Young University, who is credited with inventing ceragenins. “Further, it confirms previous findings that the compound may continue to be effective even in antibiotic-resistant strains of P. aeruginosa. While antibiotic resistance continues to be a concern, we are seeing potential, at least in this context, to find solutions.”
The study measured efficacy and the MIC of CSA-13 and tobramycin when combined with standard and tobramycin-resistant strains of P. aeruginosa. Sputum of cystic fibrosis patients was also added to each treatment to determine the impact of biofilm on each treatment.
Ceragenin Technology
N8 describes ceragenins, AKA “cationic steroidal antimicrobials” or CSAs, as “synthetic, non-peptide small molecule mimetics of endogenous host defense or antimicrobial peptides” that have broad-spectrum activity mimicking those of endogenous host defense peptides. However, they note that ceragenins are not peptides but rather synthetic small molecules that can be manufactured in large scale and are not subject to proteolytic degradation.
The company says ceragenins demonstrate antimicrobial, anti-inflammatory, immunomodulatory, and anti-cancer activity, with the antimicrobial activity extending to Gram negative and Gram positive bacteria, including multi-drug resistant strains and carbapenem resistant strains, biofilms, and fungi, with over 100 ceragenins having been synthesized to date. These compounds are effective because they have a net positive charge that is electrostatically attracted to negatively charged cell membranes of certain viruses, fungi and bacteria as well as a high binding affinity for such membranes and the ability to rapidly disrupt the target membranes leading to rapid cell death. N8 says that while CSAs have a mechanism of action also seen in antimicrobial peptides, which form part of the body’s innate immune system, they avoid many of the difficulties associated with their use as medicines.
The subject of more than 40 peer-reviewed articles, numerous patents, and several NIH funding awards, ceragenins have also been demonstrated to up-regulate other components of the human innate immune system, including anti-inflammatory, anti-cancer, wound healing, and bone growth properties naturally induced by host defense peptides.
N8 says its current primary development candidate being pursued for ceragenins is a therapeutic agent for treatment of Cystic Fibrosis patients. They note that compound characterization suggests an ideal fit to treat the lung infections that cause severe morbidity and mortality among people with CF.
Sources:
N8 Medical Inc.
Studia Medyczne
MedChemComm — Royal Society of Medicine
Image Credits:
Brigham Young University
Studia Medyczne






