Aztreonam is an antibiotic recommended in an inhaled solution form for cystic fibrosis (CF) patients with infections due to the Pseudomonas aeruginosa (P. aeruginosa) bacteria. CF is caused by a defect in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, which leads to impairment in the normal function of the lungs and pancreas. The disease is characterized by an abnormally high production of sticky mucus, and a higher propensity to respiratory infections.
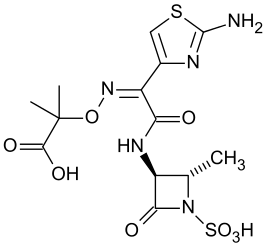
Aztreonam is a monobactam antibacterial, chemically designated as (Z)-2-[[[(2-amino-4thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid. It is currently sold in the United States under the brand names Cayston (inhaled solution) and Azactam (solution for injection or infusion).
History of Aztreonam
Bristol-Myers Squibb was the first company to receive approval by the U.S. Food and Drug Administration (FDA) to commercialize aztreonam in the U.S. as an antibacterial powder for injection or infusion, which began to be marketed under the brand name Azactam in 1998. In 2010, Gilead Sciences won FDA approval to begin commercializing aztreonam as an inhalation solution for cystic fibrosis patients with P. aeruginosa infection under the brand name Cayston. The approval was based on data from two, 28-day Phase 3 studies involving a total of 375 CF patients with P. aeruginosa age 7 years and older (CP-AI-005 and -007),with forced expiratory volume in 1 second (FEV1) of 25% to 75% predicted (mean, 55%). Results showed that aztreonam treatment significantly improved respiratory symptoms from baseline relative to placebo, with significant reductions in the bacteria’s presence in sputum observed.
 How Aztreonam Works
How Aztreonam Works
Therapy with aztreonam is administered through the inhalation of the solution, (using the using the Altera Nebulizer System, according to Gilead) which helps the antibiotic quickly enter the circulatory system and body tissues. Aztreonam is reported to have potent in vitro activity against gram-negative aerobic pathogens like P. aeruginosa. The antibiotic contains aztreonam formulated with lysine, a proprietary formulation developed specifically for inhalation.
“Although aztreonam is not well-absorbed from the GI tract, studies in rats and dogs show that absorption of nebulized aztreonam does occur via the respiratory tract,” the FDA notes in its approval documentation for aztreonam. “Respiratory tract absorption also occurs in human.”
Cayston is administered three times a day (75 mg) for a 28-day course, followed by 28 days off therapy.
Other Details about Aztreonam
Despite the safety of the treatment, side effects have been reported to occur. Less serious side effects can include mild stomach discomfort, dizziness, numbness, tingling or burning pain, mild skin rash or itching, as well as vaginal itching or discharge. According to the drug’s maker, Gilead, adverse reactions reported in more than 5 percent of Cayston-treated patients, compared to placebo patients, in the Phase 3 studies were: cough (54 percent versus 51 percent), nasal congestion (16 percent versus 12 percent), wheezing (16 percent versus 10 percent), pharyngolaryngeal pain (12 percent versus 11 percent), pyrexia (13 percent versus 6 percent), chest discomfort (8 percent versus 6 percent), abdominal pain (7 percent versus 5 percent), and vomiting (6 percent versus 4 percent).
Cayston is contraindicated in patients with a known allergy to aztreonam because of severe allergic reactions.
Note: Cystic Fibrosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
