#CHEST2016 – eRapid Device Tracks CF Patient Use of Nebulizer for Better Adherence
Written by |
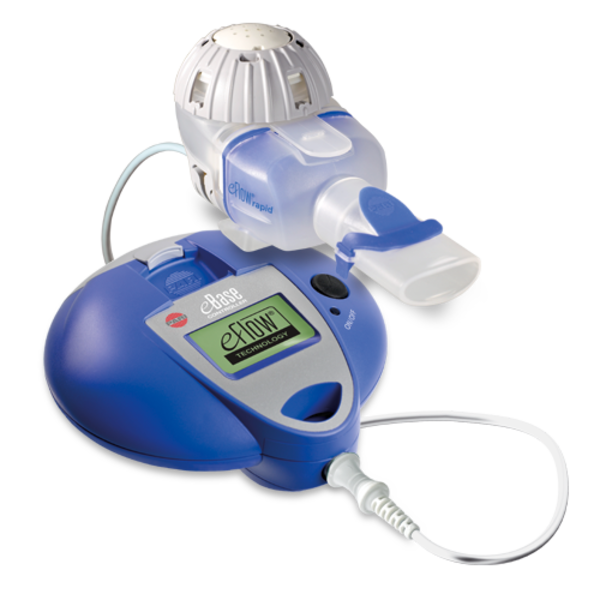
A new device using Bluetooth technology, called eRapid, can administer inhaled therapies to patients with cystic fibrosis (CF) and alert doctors that patients have taken their medicine, improving their adherence to treatments. The findings come from a study conducted by researchers at Ventura County Medical Center in California.
The study, “Improving Patient Adherence to Complex Medical Regimens Through Blue Tooth-Enabled Technology,” will be presented at this year’s CHEST Annual Meeting, in Los Angeles on Oct. 22–26.
Adherence to therapy remains low among CF patients, ranging from 31 percent to 53 percent for inhaled antibiotics, and programs to improve patient engagement have had inconsistent success. The new device shows promise in addressing this problem.
eRapid Nebulizer System. Photo: PARI
The eRapid device allows for a quick and efficient inhalation, lowering treatment burden. The feasibility of the device was evaluated in a study that included 14 CF patients, ages 8 to 32. At the study start, only 11 percent of these patients adhered to therapy, with the remaining 89 percent either moderately adhering or not using prescribed medications at all.
For 28 days, patients used the eRapid device, which consists of a Bluetooth-enabled handheld nebulizer and the monitoring tool, to inform the patients’ doctors when and if they took their medicine.
Doctors used the technology to gain feedback on compliance to medication, and also to reward patients for adherence.
eRapid use was seen to increase adherence to therapies to 80 percent, and also improved by 30 percent measures of airflow limitation and frequency of disease flare-ups.
Researchers believe that these preliminary results are encouraging, and that the eRapid device has the potential to replace pharmacy refill histories and daily phone diaries as a measure for adherence. The device can also provide accurate information that can be used with other measures.
“eRapid decreases miscommunication and misunderstanding between the patient and the provider by replacing an error-prone self-reporting method with real-time data,” said Chris Landon, the pilot study’s lead researcher, in a news release. “Ongoing assessment of the data by the health care team allows for active engagement between the patient and provider promoting shared decision making in individualized therapy and cognitive behavioral interventions.”






