Researchers Are Developing Mobile App to Help Manage, Monitor CF Patients
Written by |

A team of 12 research partners in seven European countries are engaged in a project to develop a mobile app designed to promote autonomy and help people with cystic fibrosis (CF), their families and caregivers understand the disease and monitor treatment. The researchers are, however, finding that achieving their objective is no easy task.
The project, called MyCyFAPP (EU/H2020; CORDIS Project Reference: 643806), is being coordinated by the University Hospital La Fe in Valencia, Spain and the Trondheim, Norway-based SINTEF, the largest independent research organization in Scandinavia.
According to the European Commission’s (EC) CORDIS Community Research And Development Information Service, the majority of CF patients experience lifelong pancreatic insufficiency that compromises digestion of food and absorption of nutrients, such as proteins and fat, resulting in chronic malnutrition. This can contribute to stunted growth that can be avoided only by accurate enzyme replacement therapies and close nutritional follow-up. The agency recommends enzyme replacement implementation as an early nutritional intervention to provide better nutritional status and patient welfare, helping to avoid complications associated with the disease’s development. People with CF need help to ensure they are getting adequate nutrition and the correct amount of enzyme supplementation, as well as constant reminders.
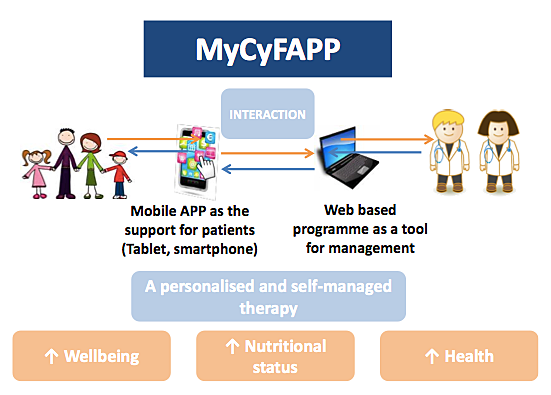
 The MyCyFAPP researchers say their portable tool will help promote a healthy lifestyle, combining patient empowerment, education and training with self-management and prevention of secondary CF-complications. SINTEF says MyCyFAPP is particularly designed to assist in self-management of enzyme replacement, enabling personalized, accurate, interactive control and monitoring of the disease by health professionals and patients, and will be clinically validated for CF self-management and monitoring.
The MyCyFAPP researchers say their portable tool will help promote a healthy lifestyle, combining patient empowerment, education and training with self-management and prevention of secondary CF-complications. SINTEF says MyCyFAPP is particularly designed to assist in self-management of enzyme replacement, enabling personalized, accurate, interactive control and monitoring of the disease by health professionals and patients, and will be clinically validated for CF self-management and monitoring.
Interviews were conducted with 71 people from seven European countries (Portugal, Spain, Italy, Germany, Belgium, the Netherlands and Norway). That group included CF patients, parents, professionals and a variety of organizations, in order to establish an overview of the needs such a mobile app must fulfill.
The MyCyFAPP researchers will develop advanced algorithms for personalized and precise enzyme dosage, and a set of information and communications technology (ICT) tools for educating patients, and for monitoring and controlling their health condition and compliance with treatment schedules.
The multidisciplinary MyCyFAPP development team includes medical experts (e.g., nutritionists, gastroenterologist and biologists) and ICT experts (e.g., human-computer interaction experts and software engineers).
Six clinical partners will conduct a multi-center clinical trial in order to assess the MyCyFAPP project solution, which incorporates advanced algorithms for adjustment of enzymes intake, medical guidelines for professionals, educational guidelines for patients, new patient-supporting, clinical and care protocols, and technology that facilitates implementation of this solution. That will include including gaming-oriented mobile and tablet applications designed to engage children, self-management mobile applications for (young) adults, and a health professional application for management of intervention strategies by healthcare service providers.
Expected benefits of MyCyFAPP app deployment are health and nutritional status improvement, leading to subsequent life quality and well-being improvement, and economic savings for the healthcare system and for patient’s families,” according to SINTEF.
The project is expected to be completed by year-end 2018.
However, SINTEF reports that the researchers have discovered that developing a digital support device to promote CF management autonomy is a challenge.
 “We knew that this work wasn’t going to be easy,” said Jacqueline Floch, leader of the work being conducted at SINTEF, in a news release. “However, the complexity of the problem still surprised us.”
“We knew that this work wasn’t going to be easy,” said Jacqueline Floch, leader of the work being conducted at SINTEF, in a news release. “However, the complexity of the problem still surprised us.”
The report notes that researchers identified an overwhelming total of 466 different and complex needs to be addressed.
“Firstly, we observe that all CF sufferers are different,” said Floch. “Some are very organized when it comes to monitoring their illness, while others are far less scrupulous. Some children may also be in denial because they don’t want to appear different. Some get a lot of support from their parents, who lay out the medicines and teach the kids how to take them, while others get almost no help at all.”
Moreover, Floch noted that the needs of individuals with CF are influenced by the disease’s gradual progression and situational circumstances — everyday needs at home and in other situations, such as at school or on holiday, when their needs will change.
The researchers also have discovered differences in respective circumstances in the countries participating in the MyCyFAPP project. For example, Norway’s efficient CF user association The Norwegian Expert Centre for Cystic Fibrosis (NFCF), has established closed groups on Facebook and also organizes a service providing peer mentors trained to support persons in similar circumstances to their own, but these systems are not found outside Norway. For instance in Belgium, some 1,200 CF sufferers are monitored exclusively by one of seven expert centers spread around the country, so a digital monitoring and management device may help reduce the disadvantages of distance between patient and healthcare personnel.
Another issue identified is that doctors, CF patients and parents have different requirements and priorities.
Moreover, in the SINTEF press release, Floch noted that the project found large variations in doctors’ enzyme dose recommendations, suggesting that overdosing may be carried out in some instances.
“We’re also seeing that many CF sufferers are looking for more than just a support device to help them calculate their enzyme doses. For example, some want an intelligent system that can make recommendations about treatment based on their own specific symptoms,” she said, adding that “doctors taking part in the project are sceptical to this suggestion. They fear that such a system will deliver incorrect advice. Our view is that the doctors’ knowledge and experience must be taken seriously and that it is important they carry out quality assurance of the work linked to the project. At the same time, it’s important that the system we develop is perceived by its users as beneficial. If not, no-one will use it,” she said.
 The MyCyFAPP researchers have categorized the needs to be addressed by the mobile app into a set of functional groups based on calculation of enzyme doses, nutritional monitoring, a daily symptoms log, treatment monitoring support, reminders, learning, digital fora and communication between CF patients and their doctors. they also have organized co-design workshops in which children aged between the ages of 4 and 15, their parents, and adult CF patients, work cooperatively on such topics as developing ideas for games and making sketches for an app designed to assist patient autonomy. Healthcare professionals including doctors, nutritional physiologists and nurses, also have participated in creating design sketches for a monitoring device.
The MyCyFAPP researchers have categorized the needs to be addressed by the mobile app into a set of functional groups based on calculation of enzyme doses, nutritional monitoring, a daily symptoms log, treatment monitoring support, reminders, learning, digital fora and communication between CF patients and their doctors. they also have organized co-design workshops in which children aged between the ages of 4 and 15, their parents, and adult CF patients, work cooperatively on such topics as developing ideas for games and making sketches for an app designed to assist patient autonomy. Healthcare professionals including doctors, nutritional physiologists and nurses, also have participated in creating design sketches for a monitoring device.
“Software development is well underway, and an initial assessment is planned for this autumn,” said Floch. “The aim is to produce software ready for a clinical evaluation in the summer of 2017.”