For CF Patients, Polymyxins Come Back Into Style To Fight Pseudomonas Aeruginosa
Written by |
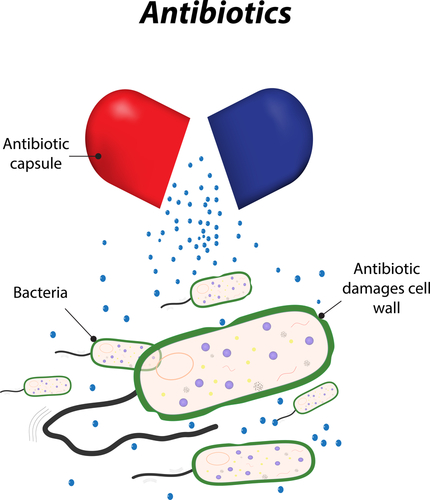
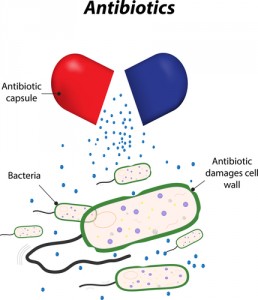 The European Medicines Agency (EMA) recently reviewed products containing the antibiotics colistin or colistimethate sodium (known as polymyxins) and recommended alterations to product sheet information to include safety and the most updated information when applied to treating infections that demonstrated to be resistant to other standard antibiotics. This update is particularly important for patients with cystic fibrosis with Pseudomonas aeruginosa, as CHMP has specifically recommended the use of colistimethate sodium for CF patients, and that the administration route should include inhalation or the use of a nebulizer.
The European Medicines Agency (EMA) recently reviewed products containing the antibiotics colistin or colistimethate sodium (known as polymyxins) and recommended alterations to product sheet information to include safety and the most updated information when applied to treating infections that demonstrated to be resistant to other standard antibiotics. This update is particularly important for patients with cystic fibrosis with Pseudomonas aeruginosa, as CHMP has specifically recommended the use of colistimethate sodium for CF patients, and that the administration route should include inhalation or the use of a nebulizer.
Due to their wide range of adverse effects, Polymyxin use has decreased since 1960 and have been substituted with other antibiotics with fewer adverse reactions. However, due to the rise of antibiotic resistance to many current antibiotics, the fact that polymyxins have not been widely used for decades means that bacteria didn’t have time to evolve resistance mechanisms to them. As such, colistimethate sodium is a viable candidate for treating infections where other antibiotics have failed, particularly in patients who may have developed resistance to other antibiotics, as is sometimes the case with CF patients. However, the renewed interest in polymyxins is bringing with it updates related to dosages and pharmacokinetics.
Accordingly, the Agency’s Committee for Medicinal Products for Human Use (CHMP) recently reviewed these medicines’ data, particularly their pharmacokinetics, effectiveness, and safety information. The review, however, included only medicines administered via injection or inhaled, leaving out all other routes of administration (orally and topically).
[adrotate group=”1″]
CHMP concluded that colistimethate sodium administration is to be restricted to patients with serious infections that have failed other antibiotics or whose treatment options are limited. However, CHMP recommends colistimethate sodium should be given together with other antibiotics whenever possible. To maximize the effect of colistimethate sodium, the antibiotic should be administered with the highest concentration possible for the treated patient, therefore maximizing its action in time. Despite limited data, CHMP issued a table of recommended doses for specific patients, mainly patients with kidney problems and in children. Additionally, it also recommended dosages for adults when the administration route is directly into fluid surrounding the brain or spinal cord. Relative to dosages, CHMP recommended that these should be issued in international units (IU) and a conversion table should be included with product information.
Commissioned by the European Union (EU), CHMP recommendations will now be evaluated, and the EU issues the final report.






