Structure of Membrane Protein That May Allow for Flow of Chloride in Cells Revealed in Study
Researchers determined the structure of a calcium-activated chloride channel, called TMEM16A, which they suggest could offer a new way of treating cystic fibrosis (CF).
The study by scientists at University of Zurich, Switzerland, reporting these findings is titled “Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM” and published in the journal Nature.
CF is caused by a defective channel due to mutations (errors) in the gene coding for the CFTR protein. The channel transports chloride and sodium, two important ions for health. In CF, improper secretion of chloride in certain cells results in the dehydration of the mucus layer in organs like the lungs.
One possible treatment approach is to activate another channel, located in the same tissues as CFTR (epithelial tissues, which line surfaces and cavities of organs and blood vessels), to re-establish the flow of chloride. One such channel is TMEM16A.
Building on the previously identified structure of TMEM16 — a member of the large family of TMEM16 membrane proteins, known to play a key role in blood coagulation — helped researchers unravel the structure of TMEM16A.
Led by Raimund Dutzler, the team combined electrophysiology with a technique called cryo-electron microscopy (cryo-EM) to find the unique features of TMEM16A and what distinguishes it from other family members.
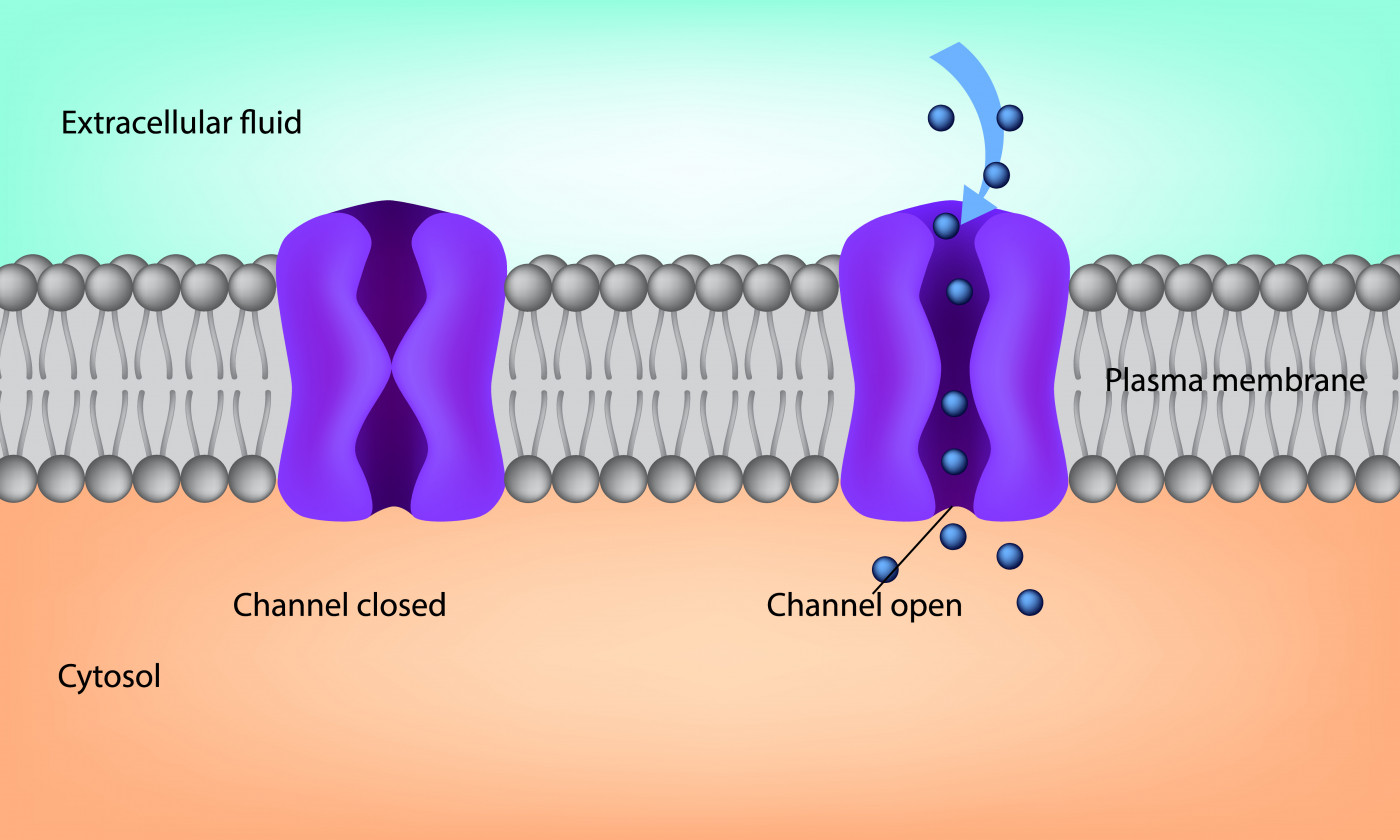
They found that the region of TMEM16A structure within the cell membrane is closed in the absence of calcium. But in the presence of calcium ions, the channel opens to allow the passage of chloride ions across the membrane.
“This activation mechanism is unique, since the bound calcium ions directly change the structure and electrostatics of the ion permeation pore,” Cristina Paulino, study’s first author, said in a press release.
Unveiling the structure of TMEM16A was seen to be a significant step in understanding how this family of membrane proteins functions, and one that could aid in future therapy development.
“The molecular architecture of this membrane protein is crucial for the targeted development of drugs for treating cystic fibrosis,” said Dutzler, the study’s senior author. “Substances leading to the activation of the TMEM16A would compensate the defect in the secretion of chloride ions in the lung.”







