UNM And Avisa Pharma Developing Fast, Inexpensive, Breathalyzer Test For CF, TB, HCAP, VAP, And Other Lung Infections
Written by |
Santa Fe, New Mexico based startup Avisa Pharma Inc. is a clinical stage company established in 2010 to develop and commercialize a new technology invented by Dr. Graham Timmins, Avisa’s Co-founder/Chief Science Officer and a University of New Mexico College of Pharmacy Associate Professor, who was assisted by colleagues from UNM’s School of Medicine. STC.UNM (STC), the technology-transfer arm of the University of New Mexico (UNM), has signed an exclusive option agreement with Avisa Pharma to license Dr. Timmons’s cystic fibrosis (CF) technology, the first rapid, biomarker-based, point-of-care breath test for the detection of serious bacterial lung infection. This breakthrough breath test technology combines two key components: AV-U13 inhalable urea solution with Avisa’a portable AVISAR SPEC (laser spectrophotometer). The AV BreathTest assesses infection in the entire lung within minutes.

Avisa’s development program objective for the breath test is to tailor the technology into a diagnostic tool for rapid monitoring of lung Pseudomonas infection and its response to antibiotic treatment in diseases such as CF. In addition to the CF related technology, Avisa has also entered into an agreement to exclusively license similar technology for Tuberculosis (TB). Both the CF and TB technologies are supported by issued patents. The follow-on targets are pathogens associated with healthcare-acquired pneumonia (HCAP), ventilator-associated pneumonia (VAP) and cystic fibrosis (CF).
Rapid detection of serious lung infections is highly desirable to improve patient outcomes, yet current techniques suffer from a number of key difficulties: Analysis often takes a long time, with bacterial cultures requiring many hours to several days. Furthermore, molecular diagnostics cannot measure the whole lung, cannot distinguish colonization from infection, are not point-of-care, and cannot monitor therapy.
[adrotate group=”1″]
Avisa observes that rapid identification of serious lung infection, such as tuberculosis, is a critical medical and public health challenge worldwide, and that rapid, point-of-care detection of these infections would significantly improve clinical outcomes and lower healthcare costs, saving millions of lives and millions of dollars worldwide.
CF patients have a genetic defect causing excessive thick mucus in their lungs that leads to chronic lung infections. Currently, no fast, effective test is available to monitor lung microbiology and response to therapy in cystic fibrosis, yet being able to do this could greatly benefit people with the disease. Avisa’s vision is to develop a diagnostic tool for Pseudomonas that would significantly improve CF management. Early, aggressive treatment of CF lung infections improves prognosis, but most CF infections are not treated until they cause increased symptoms (and more lung damage). Avisa’s technology addresses this vital gap in CF treatment by providing a rapid, point-of-care diagnostic technique.
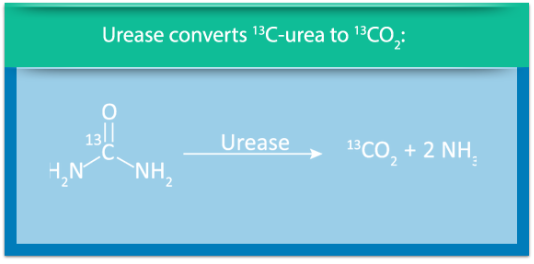
The technology developed by Dr. Timmins and Avisa assesses the bacterial load in the entire lung by measuring exhaled labeled carbon dioxide that is produced by the bacterial metabolism of an inhalable, pharmaceutical grade, synthetic urea labeled with 13C stable non-radioactive isotope of carbon — AV-U13 — the tracer component of the breath test.
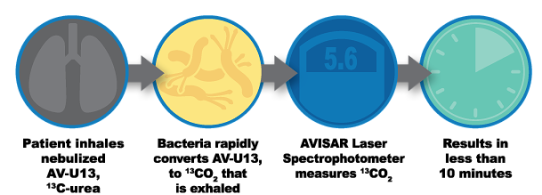
Most serious lung pathogens make urease, a unique bacterial enzyme, to convert our body’s natural urea into carbon dioxide and ammonia. The AV BreathTest exploits this reaction to detect these pathogens in the lung. AV-U13 is administered to the patient through a nebulizer in minutes, where it is metabolized in the lung by P. aeruginosa to 13CO2, which is then exhaled and easily and sensitively measured. The amount of 13CO2 exhaled is a function of the amount of infection in the lung, detected by the AVISAR spectrophotometer.
The Avisa technology is based on the labeling of a substrate that is metabolized only by bacteria and gives off CO2. It delivers the substrate directly to the lungs and measures the exhaled breath for the release of labeled CO2 which is the measure of bacterial presence. The mechanism of action utilizes detection predominantly based on known virulence factor through the use of stable, non-radioactive isotopically labeled compound breath tests of exhaled carbon dioxide for disease inducing pathogens.
A similar approach to testing exhaled breath for stable isotopes to measure bacterial colonization has been successfully used for detecting and treating H. pylori in patients with ulcers. Avisa notes that stable isotope breath testing has been a real-game changer in rapid detection and management of H. pylori infection; and they want to do the same for CF and TB.
The entire breath test process reports on urease-expressing lung pathogens from the increase in exhaled 3CO2 within minutes at the point-of-care whether it is a hospital, clinic, or out in the field.
 “The sooner we can diagnose if somebody’s ill or not,”Dr. Timmins told KOAT-TV Albuquerque’s Angela Brower, “the sooner we can intervene with the right kind of drug therapy, and the sooner those people are not going to be transmitting TB.”
“The sooner we can diagnose if somebody’s ill or not,”Dr. Timmins told KOAT-TV Albuquerque’s Angela Brower, “the sooner we can intervene with the right kind of drug therapy, and the sooner those people are not going to be transmitting TB.”
Avisa says AV BreathTest advantages include:
• Detection of bacteria throughout the entire lung
• Delivers results in minutes
• Ultra-portable and battery-powered device can work wherever needed
• No radioactive compounds are ever used
The AVISAR SPEC (laser spectrophotometer) is a rugged, portable device that rapidly measures breath13CO2/12CO2 isotope ratios. The AVISAR SPEC uses unique IR spectroscopic detection to enable a compact, battery-powered unit weighing about 8 pounds (4 kg). AVISAR SPEC’s rugged, portable design allows it to be as easily carried to remote sites in a backpack as it is around clinics or hospital departments.
According to a United States Securities And Exchange Commission FORM D Notice of Exempt Offering of Securities, Avisa has raised $3,670,716 of a targeted $4,000,000 equity issue for funding additional to a reported some $5 million previously raised, to finance continued development and clinical trials of its breath diagnostic technology and its applications.
STC.UNM (STC) is a nonprofit corporation formed and owned entirely by the University of New Mexico Regents (UNM). STC’s mission is to support UNM and its partners as the source of innovation management and commercial development. STC does this by connecting the business community to UNM; protecting and transferring intellectual property and faculty inventions to the commercial marketplace; and by assisting companies and organizations that wish to access facilities, expertise, and research conducted at UNM. As STC has evolved, the corporation has come to support not only UNM faculty, but students, outside inventors, and entrepreneurs as well.
 Lisa Kuuttila, STC President CEO & Chief Economic Development Officer, comments in a UNM-STC release: “We are very excited about this important technology being commercialized for the benefit of cystic fibrosis patients. It is an excellent example of the translation of UNM research into the clinic.”
Lisa Kuuttila, STC President CEO & Chief Economic Development Officer, comments in a UNM-STC release: “We are very excited about this important technology being commercialized for the benefit of cystic fibrosis patients. It is an excellent example of the translation of UNM research into the clinic.”
David S. Joseph, Chief Executive Officer and Co-Founder of Avisa, adds: “We chose the company’s name, Avisa, from avisar, meaning to warn in Spanish, and we believe that this early warning system can transform the way we manage this disease where the average [U.S.] lifespan is 37 years of age.”
See \ KOAT-TV Albuquerque’s news feature on the UNM/Avisa Pharma technology at:
https://www.koat.com/news/tb-diagnosis-research/29220032.
Sources:
Avisa Pharma, Inc.
University of New Mexico
STC.UNM
KOAT-TV
Image Credits:
Avisa Pharma, Inc.
University of New Mexico
STC.UNM