Patient Access to Kitabis Pak for CF Expands to More US States
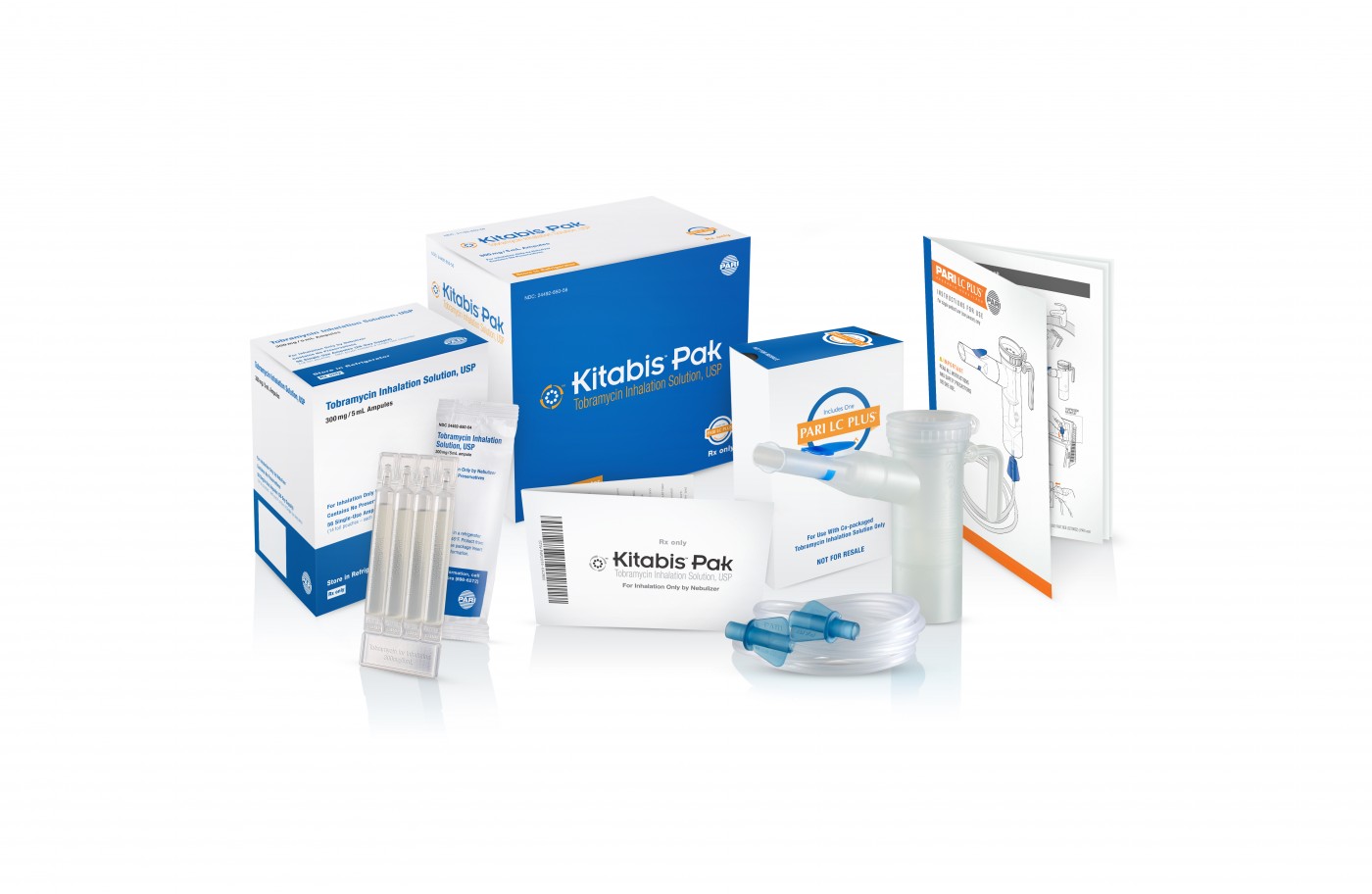
PARI Respiratory Equipment, Inc., a leading, worldwide developer and manufacturer of fast and efficient aerosol delivery systems for patients with asthma, chronic lung disease, cystic fibrosis, RSV, VAP, and HAP, recently reported on important news concerning its therapeutic products for US patients living with cystic fibrosis. Its proprietary Kitabis Pak (co-packaging of tobramycin inhalation solution and the PARI LC PLUS Reusable Nebulizer), indicated for the management of CF in adults and pediatric patients 6 years of age and older with P. aeruginosa, has been added to the Preferred Drug List (PDL) in more states across the country.
“Patients with CF that are covered by these Medicaid plans, and prescribed Kitabis Pak will have complete access to the only nebulizer handset used in the clinical trials for tobramycin inhalation solution,” said Lisa Cambridge, MSHS, RRT, director of Medical Science & Pharmaceutical Alliances at PARI. “The goal of Kitabis Pak is to increase patient access through this strategic co-packaging of both drug and device in one box.”
The Kitabis Pak has been clinically proven to be therapeutically equivalent to TOBI (tobramycin inhalation solution, USP) and carries the “AN” designation in the FDA’s Orange Book, indicating that “there are no known or suspected bioequivalence problems” associated with Kitabis Pak, according to the FDA. Cystic Fibrosis News Today previously reported on how the U.S. Food and Drug Administration (FDA) has classified Kitabis Pak in its Orange Book (an FDA resource listing approved drug products with therapeutic equivalence evaluations) as a therapeutic equivalent to TOBI (tobramycin inhalation solution, USP) for cystic fibrosis (CF).
Each Kitabis Pak contains a 28-day treatment of generic tobramycin inhalation solution and the PARI LC PLUS nebulizer handset, which is currently the only device approved by the FDA for the delivery of tobramycin inhalation solution.
“Expanded patient access to Kitabis Pak is welcome news for both CF patients and healthcare professionals,” says Mary Lester, RRT, RCP. “A new LC PLUS nebulizer handset co-packaged with each course of drug not only improves patient access to the right device, but also helps maintain drug delivery and address the challenges of nebulizer maintenance and acquisition.”
To learn more about the Kitabis Pak and how to purchase, visit www.Kitabis.com.







