AffloVest Airway-Clearance Device, for Patients with Cystic Fibrosis and Other Respiratory Diseases, Now Made in America
Written by |

 Austin, Texas-based International Biophysics Corporation, maker of AffloVest — a completely self-contained, battery operated, portable high-frequency chest wall oscillation
Austin, Texas-based International Biophysics Corporation, maker of AffloVest — a completely self-contained, battery operated, portable high-frequency chest wall oscillation  (HFCWO) device for bringing critical therapy to people struggling with cystic fibrosis (CF), bronchiectasis, and other respiratory diseases — is joining several major U.S. companies such as Apple in bringing the manufacture of one of its main products home to America.
(HFCWO) device for bringing critical therapy to people struggling with cystic fibrosis (CF), bronchiectasis, and other respiratory diseases — is joining several major U.S. companies such as Apple in bringing the manufacture of one of its main products home to America.
While it has made economic sense for U.S. companies to manufacture offshore in recent years to take advantage of lower labor costs, tax advantages, and other cost savings, International Biophysics Corp. observes that recently, the “here vs. there” differential has been leveling.
In a press release, International Biophysics Corp. CEO David Shockley Jr. said, “We manufactured the AffloVest in Germany, but overall costs in Europe, like China, kept rising.”
That change bolstered the advantages of “Made in the USA” branding, which includes positives such as U.S. manufacturing facilities, workers’ productivity, and quality performance being among the highest in the world.
“By on-shoring our manufacturing we are creating great jobs domestically, made possible by improved quality and cost competitiveness. We all win,” Shockley said.
International Biophysics Corporation’s marquee product, AffloVest, is a cutting-edge technology airway clearance vest that represents a major advance in the treatment of respiratory diseases such as CF, chronic obstructive pulmonary disease (COPD), chronic bronchitis, and similar related ailments.
AffloVest provides respiratory patients with new freedom of mobility during treatments. German-engineered and already widely adopted in Europe, AffloVest is marketed in the U.S. by International Biophysics Corp.
An estimated 25,000 to 30,000 children and adults in the U.S. (70,000 worldwide) have cystic fibrosis, with approximately 1,000 new cases diagnosed each year. Additionally, there are more than 10 million people diagnosed with COPD, according to the Centers for Disease Control and Prevention.
People with cystic fibrosis have difficulty clearing pathogens from the lung and experience chronic pulmonary infections and inflammation. There is no cure, but survival ages have increased along with major advances in treatment.
The new “Made in America” AffloVest features several innovations distinguishing it from the previous design, including a better overall tailored fit; a fashionable denim color; an electronic digital programmable controller with compliance monitoring; longer battery life; and more sizes. International Biophysics is also introducing a new AffloVest Pro, a special infection control disposable enhanced hospital version that gives healthcare professionals greater oscillation control to target specific parts of the lungs.
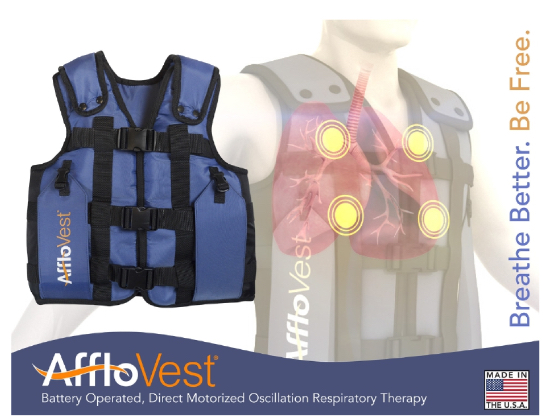
Before, airway clearance vests tethered the patient by an air hose to bulky machines, which require direct electrical current. But AffloVest features built-in oscillating compressor modules and claims to be the first-ever portable, battery-operated HFCWO vest, enabling users to move about freely during treatments, or, if they prefer, to sit comfortably in almost any environment. Users can go for a walk outside, move around the house, or relax in front of the television during therapy.
 The patient slips the vest on, buckles three clips on the front of the vest, tightens the straps to ensure a secure fit, and then, depending on individual treatment needs, chooses one of nine settings using a handheld control attached to the right side of the vest. A rechargeable battery pack with several hours of charge life is fixed to the right side of the vest and provides the required amount of power for mobile treatments.
The patient slips the vest on, buckles three clips on the front of the vest, tightens the straps to ensure a secure fit, and then, depending on individual treatment needs, chooses one of nine settings using a handheld control attached to the right side of the vest. A rechargeable battery pack with several hours of charge life is fixed to the right side of the vest and provides the required amount of power for mobile treatments.
The AffloVest also includes an A/C to D/C power adapter that allows the vest to be powered indefinitely from wall current if longer treatment periods are required, or a charged battery unit is not available for use. When fully charged, the battery has the capacity for more than six treatments at maximum intensity. Treatment times can vary depending on physician prescription, but they generally take 20-30 minutes, twice a day.
International Biophysics Corp. says AffloVest, which is available in seven sizes (XXS, XS, S, M, L, XL, XXL) to serve both children and adults with chronic lung conditions, and weighs in at approximately 10 pounds, is the lightest and quietest device of its kind on the market today, and unlike other respiratory vest options, it provides adjustable levels of treatment intensity with three modes of oscillation treatment (Drainage, Vibration, Percussion), and utilizing all three therapies in a treatment session enhances successful airway clearance.
Doctors and patients can tailor their treatments to their needs, with settings that provide chest wall oscillation treatment on both the front and back of the vest. Three adjustable intensity levels (soft, medium, intense) and nine setting variations let patients choose their own level of treatment. The AffloVest’s frequency ranges from 5 Hz to 20 Hz. The intensity levels dictate the frequency, which generally runs at 5 Hz for the lowest setting, 13 Hz for the medium setting, and 20 Hz for the highest setting. AffloVest is quiet during operation.
 Because of AffloVest’s unique integral module system, the treatment does not vary with the patient’s position, and can be administered while the patient is sitting, standing, or laying down. Being easy to store and easy to carry, International Biophysics Corp. says AffloVest is an ideal solution for respiratory patients who travel.
Because of AffloVest’s unique integral module system, the treatment does not vary with the patient’s position, and can be administered while the patient is sitting, standing, or laying down. Being easy to store and easy to carry, International Biophysics Corp. says AffloVest is an ideal solution for respiratory patients who travel.
HFCWO therapy involves rapid oscillation of the patient’s chest wall, using pneumatic energy to promote a more productive cough in the patient. Clinical studies on AffloVest revealed that its users experience improved lung function, attributed to AffloVest’s motor-driven, directed oscillation therapy and its portability and ease of use, which increases patient compliance.
One such study, commissioned by International Biophysics Corp. and conducted by Michael Cooper, a registered respiratory therapist in Chicago at a leading pediatric respiratory care clinic, found that the device improved several lung function parameters by more than 10 percent using direct percussion as well as the vibration.
The HFCWO technique has been demonstrated to effectively promote the mobilization of bronchopulomary secretions over a control group. A March 2013 clinical randomized efficacy study commissioned by International Biophysics Corp. and conducted by Dr. Konrad Schultz, chief physician at the Bad Reichenhall Clinic in Munich, Germany, investigated usage benefit of the AffloVest as an HFCWO treatment device.
The researchers found that before introduction of the AffloVest, and with just the implementation of the existing pulmonary and orthopedic regimen of care, 48.5 percent percent of patients studied had relatively fluid bronchial secretions (landing somewhere between 0-2 on the scale) while 51.5 percent of the patients had much thicker bronchial secretions (falling between 3-5 on the scale). Additionally, only 42.5 percent of patients were able to cough productively (again, scoring between 0-2) while 57.5 percent had relatively fewer productive coughs or a near inability to cough at all (scaled between 3-5).
These figures demonstrated that the active comparator treatment being implemented at the clinic was somewhat effective in just under half of the patients receiving care. However, after introduction of AffloVest and implementation of HFCWO, 75 percent of patients had relatively fluid bronchial secretions (landing somewhere between 0-2 on the scale) and only 25 percent of the patients had thicker bronchial secretions (falling between 3-5 on the scale). Similarly, 75 percent of patients found they were able to cough more productively (scaled 0-2) after the AffloVest treatments, and only 25 percent had fewer productive coughs.
Schultz’s study concluded that, “With every single patient, a dramatic improvement in cough productivity and bronchial secretion viscosity was observed in combination with the pulmonary and orthopedic regimen of care. It became fundamentally easier for the patients to cough out mucus, because of the more fluid secretions and more productive cough provided by the introduction of High Frequency Chest Wall Oscillation treatment with the AffloVest.”
AffloVest has been approved for Medicare and private health insurance reimbursement, earning FDA 510k market clearance in the U.S. and private health insurance reimbursement under the Healthcare Common Procedure Coding System (HCPCS) code E0483 “High Frequency Chest Wall Oscillation,” thereby opening the door for Americans with respiratory disease to benefit from the vest’s new technology and greater comfort during treatments.
AffloVest requires a prescription from a physician and is available to U.S. patients through Durable Medical Equipment (DME) and Home Medical Equipment (HME) retailers.
For more information, visit https://www.afflovest.com/