Kitabis Pak for Cystic Fibrosis Listed in Top 10 Notable Drug-Device Approvals of 2014
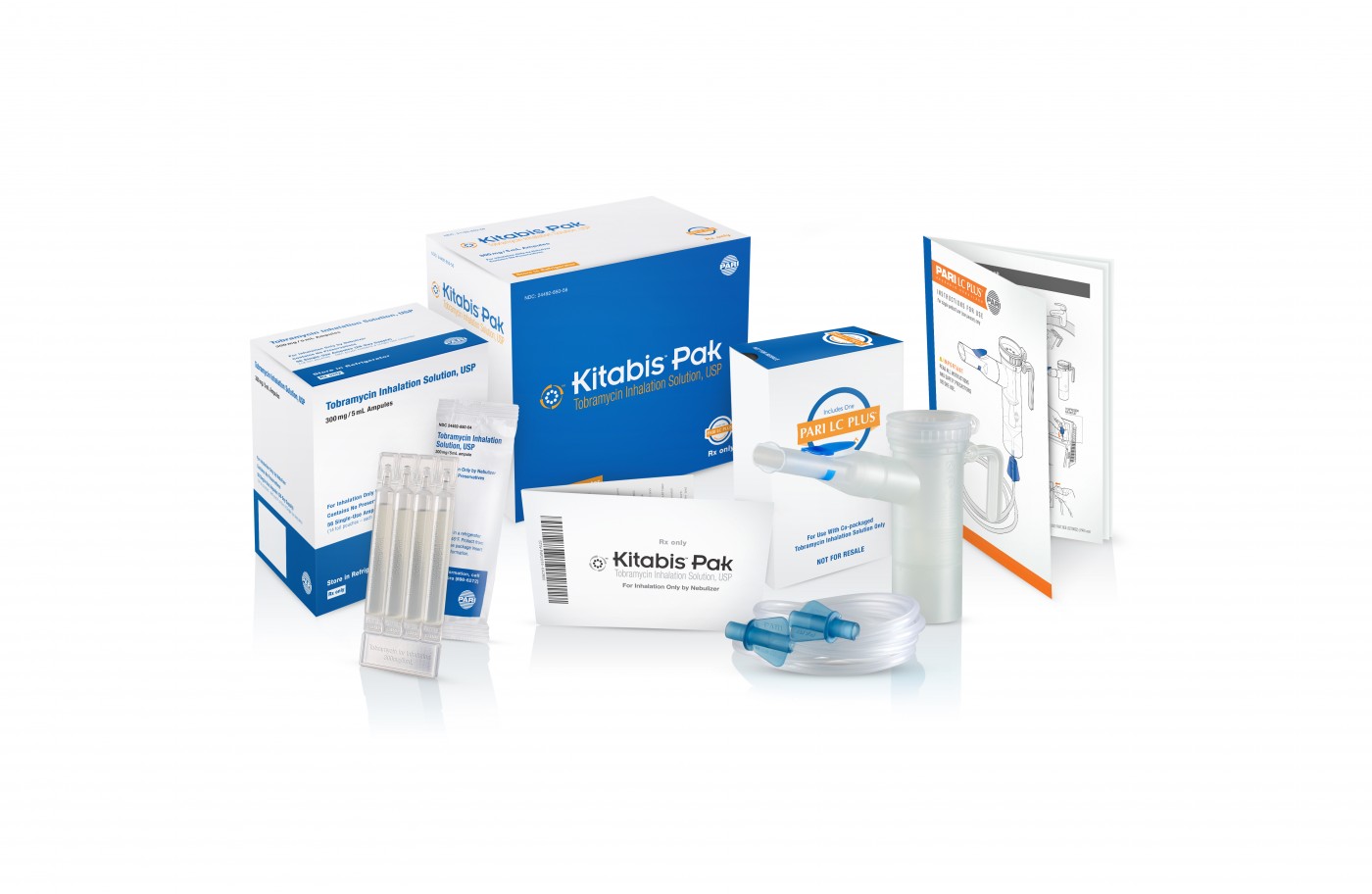
The first-of-its kind Kitabis Pak, a co-packaged generic tobramycin inhalation solution and PARI LC PLUS Nebulizer indicated for cystic fibrosis, was recently named one of 2014’s 10 notable drug-device approvals. The list is currently published in this month’s issue of Drug Development & Delivery.
The Kitabis Pak has been clinically proven to be therapeutically equivalent to TOBI (tobramycin inhalation solution, USP) and carries the “AN” designation in the FDA’s Orange Book, indicating that “there are no known or suspected bioequivalence problems” associated with Kitabis Pak, according to the FDA.
Each Kitabis Pak contains a 28-day treatment of generic tobramycin inhalation solution and the PARI LC PLUS nebulizer handset, which is currently the only device approved by the FDA for the delivery of tobramycin inhalation solution.
“Having Kitabis Pak recognized as a notable drug-device approval confirms PARI’s vision to make the prescription process for generic tobramycin and the PARI LC Plus nebulizer more convenient and cost effective for patients,” said Geoff Hunziker, president of PARI USA. “Since Kitabis Pak is one prescription for both the drug and nebulizer handset, it ensures patients receive the proper delivery device for tobramycin inhalation solution.”
“We have always believed that it is important for patients and healthcare professionals to understand that tobramycin inhalation solution is only approved for use with the nebulizer handset in Kitabis Pak,” said Jan Zimmermann, Portfolio Manager at PARI USA. “Being recognized in this category alongside other innovative pharmaceutical companies confirms our approach in setting a new standard of care for nebulized drugs.”
It is well documented that CF patients are resigned to a strict daily regimen of prescription drugs, medical devices and supplements. The approval of new therapeutic technologies like the Kitabis Pak are important for those with cystic fibrosis as it aids them in making the management of the disease easier, improving patience compliance with treatment.
In earlier news on the Kitabis Pak, PARI reported on how the technology had been added to the Preferred Drug List (PDL) in more states across the country.







