FDA Clears Westmed’s Vibralung Acoustical Percussor For CF, Other Respiratory Diseases
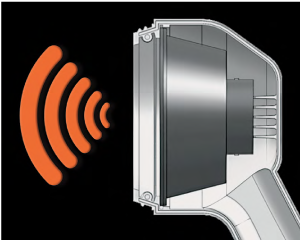
 The Food and Drug Administration (FDA) has granted market clearance to Westmed’s Vibralung Acoustical Percussor. The device was produced by the medical device manufacturing company to treat not only patients with respiratory diseases, but also other conditions and diseases that feature an increase in mucus production, secretion infection and inspissation, and defects in mucociliary clearance, such as cystic fibrosis.
The Food and Drug Administration (FDA) has granted market clearance to Westmed’s Vibralung Acoustical Percussor. The device was produced by the medical device manufacturing company to treat not only patients with respiratory diseases, but also other conditions and diseases that feature an increase in mucus production, secretion infection and inspissation, and defects in mucociliary clearance, such as cystic fibrosis.
The device works by producing vibratory sound waves, applying them during inspiration and exhalation across a broad spectrum of frequencies (from 5 to 1,200 Hz). The percurssor effectively vibrates the column of gas present in the tracheobronchial tract in order to release the mucus and separate it from the airways, which the company assures promotes gentle, safe, and effective Airway Clearance Therapy (ACT).
Westmed announced in a recent press release that the Vibralung Acoustical Percussor is safe to be used as sole or adjunct therapy, according to the patient’s needs and doctor’s indications. It also uses Positive Expiratory Pressure (PEP), which is applied simultaneously to improve the device’s benefits. Aerosol therapy with Westmed’s approved Circulaire II Hybrid high-efficiency aerosol drug delivery system is also an add-on possibility, which can be used to disperse respiratory medications concomitantly. The device is lightweight, portable, and battery-powered.
The Vibralung Acoustical Percussor may be indicated for patients diagnosed with cystic fibrosis, chronic bronchitis, bronchiectasis, pneumonia, ciliary dyskinesia syndromes, asthma, muscular dystrophy, post-operative atelectasis plus neuromuscular respiratory impairments, thoracic bellows defects, or any other cardiorespiratory or neuromuscular diseases that inhibit effective cough, mucokinesis, airway clearance, and expectoration.
[adrotate group=”1″]
Following studies on the device, the therapy may also be used to create airway clearance in cases where other medical devices cannot be used, as in cases of burns, chest wall injuries, broken or injured ribs, and recent surgical wounds. The percussor does not make contact with the external chest wall, which means it is a gentler form of ACT than oscillatory PEP devices.
The results of the clinical trials performed by Westmed, and conducted at the University of Arizona Medical Center Tucson in collaboration with the physicians at the Cystic Fibrosis Center, were presented at the NACF conference in October of 2013 in Salt Lake City.