Tobramycin-Nebulizer Kitabis Pak FDA-Approved for Cystic Fibrosis
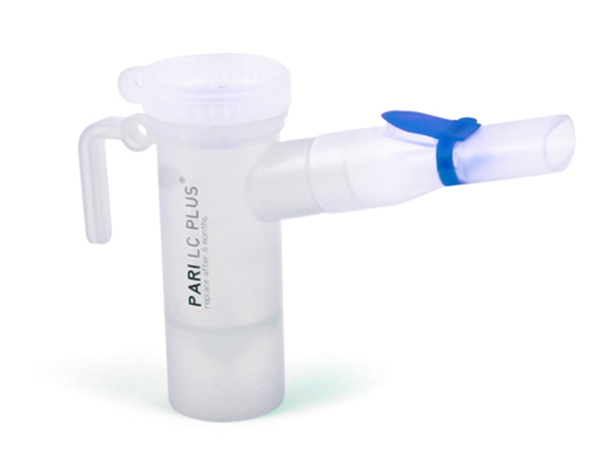
 Virginia-based surgical and medical instrument manufacturer PulmoFlow, Inc. announced Tuesday, December 2 that the US Food and Drug Administration has granted final approval of its New Drug Application for the Kitabis Pak. The drug is a co-packaging of tobramycin inhalation solution and the PARI LC PLUS® Reusable Nebulizer, which is the first combination of its kind approved and indicated for the treatment of respiratory infections caused by Pseudomonas aeruginosa in adult and pediatric patients with cystic fibrosis (CF). The Kitabis Pak is exclusively available through PARI Respiratory Equipment, Inc., a biotech company that develops aerosol delivery systems for various pulmonary conditions such as asthma, chronic lung disease, CF, RSV infections, VAP, and HAP.
Virginia-based surgical and medical instrument manufacturer PulmoFlow, Inc. announced Tuesday, December 2 that the US Food and Drug Administration has granted final approval of its New Drug Application for the Kitabis Pak. The drug is a co-packaging of tobramycin inhalation solution and the PARI LC PLUS® Reusable Nebulizer, which is the first combination of its kind approved and indicated for the treatment of respiratory infections caused by Pseudomonas aeruginosa in adult and pediatric patients with cystic fibrosis (CF). The Kitabis Pak is exclusively available through PARI Respiratory Equipment, Inc., a biotech company that develops aerosol delivery systems for various pulmonary conditions such as asthma, chronic lung disease, CF, RSV infections, VAP, and HAP.
Geoff Hunziker, the President of PARI USA, said, “Our goal is to streamline the process so patients receive tobramycin inhalation solution with the nebulizer proven to deliver it effectively. Kitabis Pak is the easiest and most cost effective way for doctors to prescribe nebulized tobramycin without the added hassle of a separate nebulizer prescription or cost.”
The Kitabis Pak will retail for the same price as the generic tobramycin alone, and is a pioneer combination product in the new standard of pairing prescribed nebulized drugs with their respective inhalation device. Cheryl Velotta, an LPN/CRT of UH Rainbow Babies and Children’s Hospital, anticipates significant benefits from the Kitabis Pak for both healthcare providers and patients, as it ensures the right drug is properly administered through the right delivery system.
[adrotate group=”1″]
According to Dr. Bruce Montgomery, the former Executive Vice President of Research and Development for TOBI® (tobramycin), the FDA based its final approval on the PARI LC PLUS Nebulizer’s proven safety and efficacy in respiratory therapy, as it maximizes drug delivery, minimizes waste, and has an average treatment time of 6-7 minutes, according to Pari’s own specs. It is reusable for up to 6 to 12 months, and is easily sanitized via boiling, dishwasher, or autoclave. Several pharmaceutical companies have partnered with PARI for approval of their aerosol medications, such as AstraZenica for Pulmicort Respules, Sepracor for Xopenex, Dey for AccuNeb and DuoNeb, and Genentech for Pulmozyme.
PARI will also offer a compressor access program called, “PARI PROVIDE,” to assist patients without access to the proper drug delivery device for their prescribed tobramycin inhalation medication.







