New CF Airway Clearance Monitoring System, inCourage, Promotes At-Home Treatment

![]() St. Paul, Minnesota-based RespirTech, specialists in the design and manufacture of Airway Clearance Therapy (ACT) vest devices for persons with lung disorders, recently announced the discovery of a novel way to remotely monitor the treatment of cystic fibrosis (CF) patients.
St. Paul, Minnesota-based RespirTech, specialists in the design and manufacture of Airway Clearance Therapy (ACT) vest devices for persons with lung disorders, recently announced the discovery of a novel way to remotely monitor the treatment of cystic fibrosis (CF) patients.
Work leading to the innovations was part of a project funded by a Small Business Innovation Research (SBIR) grant from the National Institutes of Health (NIH). SBIR grants provide early stage research and development capital for projects with strong potential for commercialization.
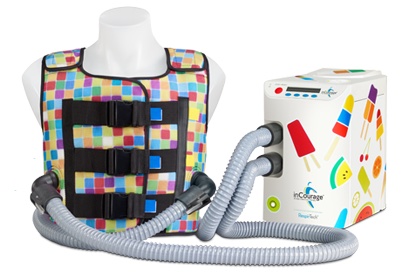
Two key diagnostic and treatment tools used in CF management — spirometry and high-frequency chest compression (HFCC) — are now, for the first time, part of a coordinated system that wirelessly transmits therapy data to a smartphone and shares it with healthcare providers via a web-based app, supplying clinicians with a more complete and accurate profile of a patient’s health status. Spirometers are devices that measure lung function, while HFCC systems — in this case, the inCourage System from RespirTech — help clear excess mucus from patients’ airways.
“By monitoring patients’ lung function and therapy adherence at home, clinicians can optimize patient care,” said RespirTech CEO, K. James Ehlen, MD, in a press release. “A primary goal of this initiative is to use ongoing data to detect and address pulmonary issues before they require more serious and costly interventions.”
RespirTech’s innovative home monitoring system enables clinicians to monitor current data, allowing them to quickly identify and respond to changes in pulmonary function, and to adjust their prescribed HFCC regimen and lung function monitoring based on the person’s specific needs. Remote monitoring is expected to be particularly helpful for patients who live in areas with limited access to CF specialists.
RespirTech notes that HFCC is a standard airway clearance intervention, and that more than 70 percent of American adults and nearly 90 percent of children ages 6-17 with CF use HFCC vest therapy, with more than 95 percent of the nation’s 110 Cystic Fibrosis Foundation-accredited CF care centers prescribing HFCC for their patients.
Other collaborators on the research project include leading pulmonary researchers affiliated with two major U.S. academic medical centers, and Health Factors, Inc., a Minneapolis company creating and implementing the connected-device strategy.
“From a patient perspective, there will now be a level of support that didn’t exist before,” said Dan Spors, Health Factors’ co-founder. “The patient does the same therapy, but the care staff will have new information and greater visibility into what the patient is doing. They will be able to correlate symptoms and objective device information, providing better context for making decisions.”
RespirTech has agreed to license resulting technologies from Koronis Biomedical Technologies, the grant applicant and an R&D firm in Maple Grove, Minnesota.
“We look forward to applying these technologies for patients with CF and other chronic airway conditions like COPD [chronic obstructive pulmonary disease] and bronchiectasis. Measures that lead to better therapy adherence and more robust treatment information can contribute to better outcomes,” said Dr. Ehlen. “Research shows that patients who adhere to therapy may experience fewer respiratory infections (1)* and a reduced need for antibiotics (2). Improved outcomes are good for patients, their caregivers and the health care system.”
It is estimated that more than 35 million people in the U.S. alone suffer from chronic lung diseases, such as CF, bronchiectasis, COPD, chronic bronchitis, and a variety of pulmonary conditions resulting from neuromuscular disorders, all of which can benefit from airway clearance therapy.
Lifelong daily airway clearance therapy is a medical necessity for CF patients to minimize the risk for recurrent infections, bacterial colonization and progressive lung disease. Significant airway inflammation may be present even in infants and young children with CF, who have minimal or no evidence of lung disease. Benefits of airway clearance therapy are greatly diminished if treatment is deferred until significant lung disease develops.
 A normal balance of mucus secretion and removal best ensures a stable and effective defense against outside elements. Airway mucus traps bacteria and other inhaled particles, and cilia move it out of the lungs. However, when the unique interaction of mucus, cilia and coughing are ineffective in clearing mucus, respiratory difficulties soon follow. Chronic effects of excess or uncleared airway secretions are well-understood, and strongly correlate with episodes of acute illness and progressive, often meaningful declines in pulmonary function. Uncleared secretions can promote chronic inflammation and infection. If unmanaged, it can result in irreversible lung damage and respiratory function impairment.
A normal balance of mucus secretion and removal best ensures a stable and effective defense against outside elements. Airway mucus traps bacteria and other inhaled particles, and cilia move it out of the lungs. However, when the unique interaction of mucus, cilia and coughing are ineffective in clearing mucus, respiratory difficulties soon follow. Chronic effects of excess or uncleared airway secretions are well-understood, and strongly correlate with episodes of acute illness and progressive, often meaningful declines in pulmonary function. Uncleared secretions can promote chronic inflammation and infection. If unmanaged, it can result in irreversible lung damage and respiratory function impairment.
Vest Therapy
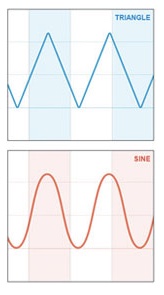
RespirTech is the developer, manufacturer, and distributor of the inCourage System, which is designed for simple, effective airway clearance therapy (ACT) for a wide range of chronic lung conditions, including CF, COPD, and bronchiectasis, an often under-diagnosed condition (3) affecting an estimated 475,000 people in the U.S. (4). RespirTech say it is the only ACT vest maker to use triangle wave air pulses that have been demonstrated to improve airway secretion clearance by as much as 20 percent over sine wave vest therapy systems (5).
Vest therapy, also known as HFCC/HFCWO, was developed, patented, and introduced in the early 1990s at the University of Minnesota by pediatric pulmonologist Warren Warwick, MD, and Leland Hansen, MPH, to provide more effective secretion clearance for children with CF. Since then, Warwick and Hansen’s technology has been well established as a safe and effective therapy in more than 80 clinical studies. HFCC is recognized as standard of care ACT for CF and other lung disorders characterized by impaired mucus clearance — a status based upon a large body of peer-reviewed and other published research, including papers and abstracts presented at major medical conferences, as well as more than two decades of clinical experience demonstrating that vest therapy is a superior airway clearance method.
Lou and Pam Mertz, parents of a CF child who was a patient at the University of Minnesota CF Center, were approached by the center’s director, Dr. Warwick, with an improved design of vest therapy — new technology that they perceived as an important care advance for children. The Mertzes, along with founding CEO Mario Nozzarella, started RespirTech in 2004, and since then, the organization has served thousands of CF and other patients with secretion clearance needs.
RespirTech’s inCourage System is designed for easy operation. Users can choose from pre-programmed therapy cycles or create and save individualized sessions. With inCourage’s Active Venting feature, taking a deep breath during therapy is also said to be more comfortable than with other vest therapy systems. The company maintains that how the system is designed makes all the difference.
RespirTech explains that most ACT vest systems use sine wave technology that increases pressure when a patient takes a deep breath — making the vest therapy feel constrictive. The Triangle Waveform of the inCourage System is different, using a unique air-chopping valve that delivers a thump to the chest to loosen, thin and move mucus out of the airways.
inCourage Airway Clearance Therapy uses air pressure and pulses to create beneficial compressions. However, unlike other ACT vest therapies, the inCourage System lets a patient’s chest expand easily for deep, comfortable breathing during therapy.
Active Venting also allows the vest to maintain a constant pressure when the patient takes a deep breath, so that it feel less restrictive and allows for deeper breathing. RespirTech’s colorful vest designs also encourage patients to adhere to daily therapy regimens, which can mean better outcomes for chronic airway clearance conditions.
*Notes 1-5: References available upon request from RespirTech.
For more information, visit www.respirtech.com.
Sources:
RespirTech