Low Level of Zinc Ions in Lungs Contribute to Buildup of Mucus in CF, Study Finds
When two channels that are supposed to move chloride and sodium ions out of cells in the lungs fail to function properly, it leads to the mucus buildup seen in cystic fibrosis.
Japanese researchers have discovered that the channel dysfunctions also reduce the amount of zinc ions going into the lungs, further contributing to the thick mucus accumulation.
Their study, published in the journal EBioMedicine, is titled “Zinc Deficiency via a Splice Switch in Zinc Importer ZIP2/SLC39A2 Causes Cystic Fibrosis-Associated MUC5AC Hypersecretion in Airway Epithelial Cells.”
The Kumamoto University research dealt with the CFTR and ENaC channels. A malfunctioning CFTR channel fails to deliver enough chloride ions to the lungs. Meanwhile, a hyperactive ENaC channel leads to the delivery of too many sodium ions. Both malfunctions contribute to the mucus buildup.
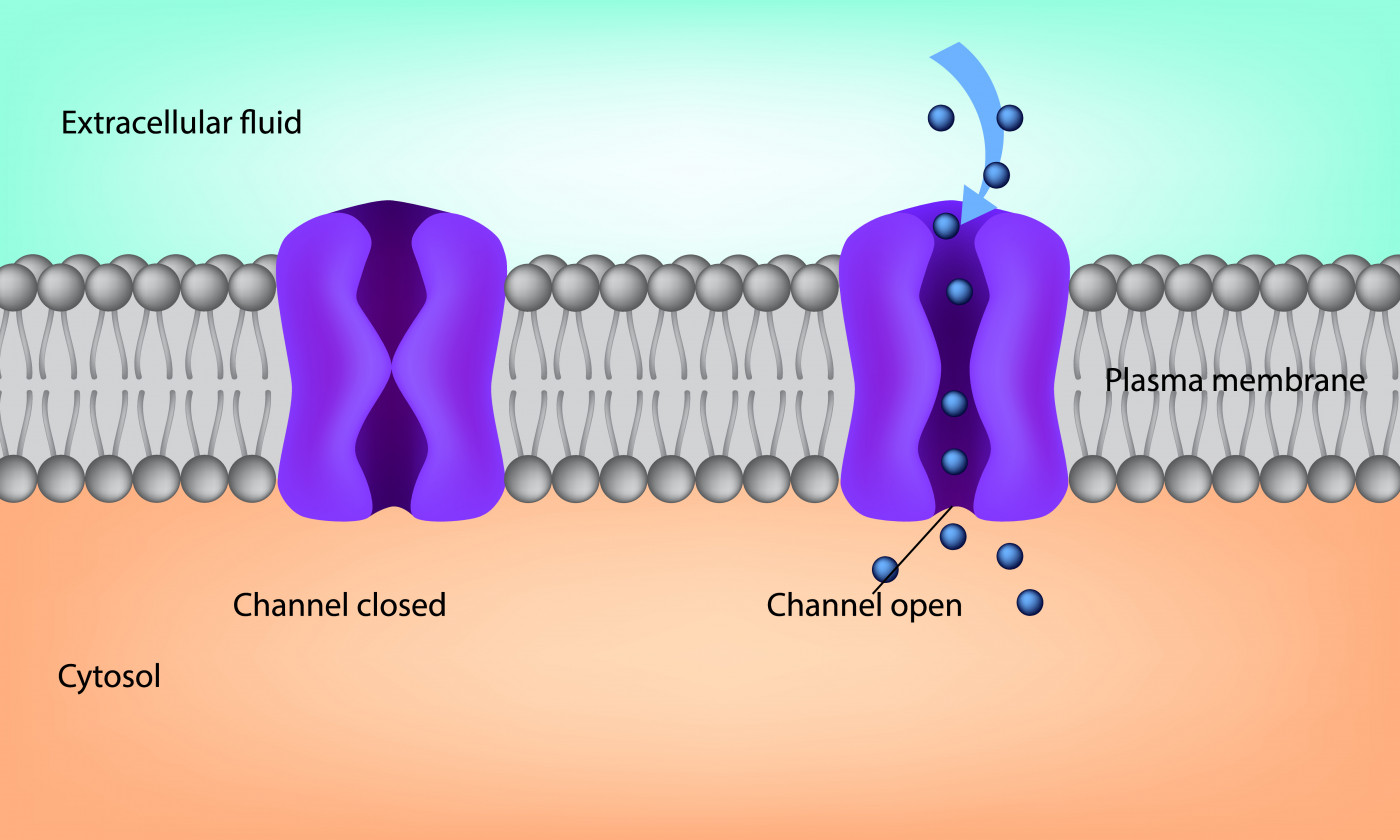
Normally functioning ion channels, or transporters, ensure balanced airway mucus production.
A mutation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene disrupts the transporter’s delivery of chloride ions — one of the main contributors to cystic fibrosis.
Hyperactivation of the second transporter, the epithelial sodium channel (ENaC), also contributes to mucus building up in airways. The accumulation not only reduces airway clearance, making it difficult to breathe, but creates an environment that promotes bacterial infections.
Although scientists know that the CFTR channel transports chloride ions and the ENaC channel sodium ions, they have known little about other ions’ role in the development of CF.
The Kumamoto team decided to see if zinc ions play a role as well.
Previous studies gave them tantalizing hints. The research showed that “zinc levels are dysregulated (lower in blood and higher in sputum) in CF patients,” the team wrote. The studies also demonstrated that zinc supplements can improve CF patients’ respiratory function and lower their infection rates, the team added.
Their research showed that lung epithelial cells with a defective CFTR channel or hyperactive ENaC channel have lower than normal levels of zinc.
The team also discovered that lower zinc levels contribute to increased production of a protein known as MUC5AC that is found in high levels in the airways of people with CF. Overproduction of MUC5AC is associated with the mucus buildup in the airways that creates the perfect environment for bacterial infections.
Overall, “our data unveil a previously unknown connection between intracellular Zn2+ [zinc] and mucus production at the cellular level of CF airways,” the team wrote. The finding could lead to the development of a treatment for the disease that revolves around zinc ions, they wrote.







