Vertex’s Kalydeco May Aid Insulin Secretion in CF Patients
Written by |
A small study by scientists in Israel indicates that treatment with ivacaftor in people with cystic fibrosis (CF) and a specific gene mutation may help with insulin secretion problems. The study, titled “CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation,“ appeared in the Nov. 4, 2015, issue of the Journal of Cystic Fibrosis.
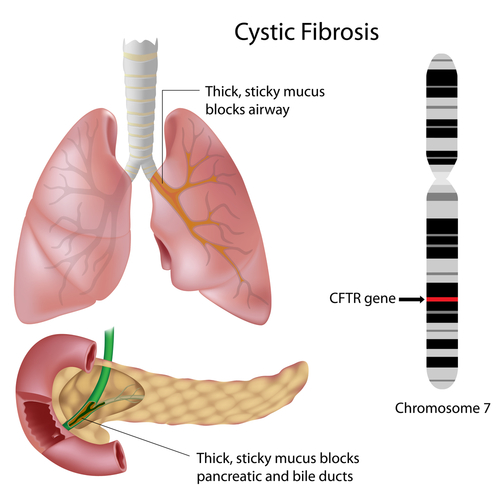
The Cystic Fibrosis Foundation and Vertex Pharmaceuticals developed ivacaftor (known by its trade name Kalydeco), which is the first treatment that targets the cause of CF, rather than just the symptoms. The therapy is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator. The CFTR allows chloride and thiocyanate to cross cell membranes. When the CFTR does not work correctly, problems with fluid transport in the lung, pancreas and other organs results, causing CF. Ivacaftor helps to normalize this chloride and thiocynanate “gating” across cell membranes, reducing the symptoms of CF.
Ivacaftor is indicated for use in people with specific CF mutations, including G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, S549R or R117H. The current study demonstrated that insulin secretion improvement may occur specifically in those people with the S549R mutation. It remains to be seen whether it is effective for impaired insulin secretion in people with other CF mutations.
CF is one of the most commonly occurring chronic diseases of the lungs in children and young adults, and can be a life-threatening disorder. Breathing is often difficult in cystic fibrosis due to a sticky mucus that builds up in the lungs and all too often leads to serious bacterial lung infections. CF can also cause other health problems, including problems with the pancreas and insulin regulation.
Led by Reuven Tsabari of the Division of Pediatric Pulmonology and CF center in Jerusalem, Israel, the current study involved 16 weeks of treatment with ivacaftor in two CF patients, siblings who carry the S549R gating mutation. The researchers measured their glucose and insulin levels.
Ivacaftor treatment improved insulin secretion patterns and also lowered blood glucose. Because this study only involved two people with CF, additional work with more CF patients is needed for conclusions to be drawn.
As the scientists noted, “Further studies and larger cohorts are needed to evaluate the impact of ivacaftor on insulin secretion in patients with CF carrying gating or other mutations.”
It will be of particular interest to see if ivacaftor can similarly improve insulin secretion in people with other types of CF-associated mutations.






