Data, Published by Company, Shows AffloVest Device Helps CF Patients with Airway Clearance
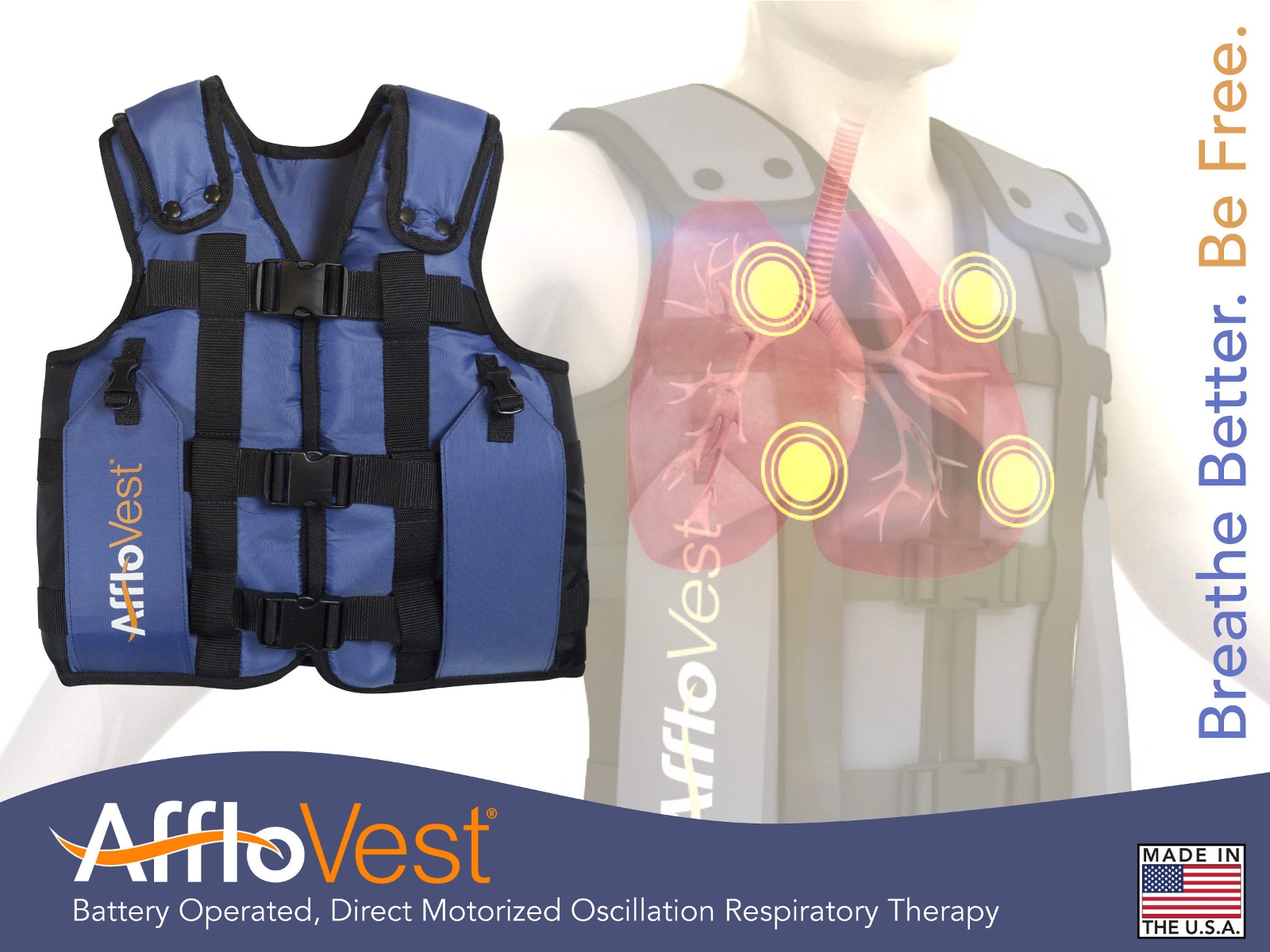
(Image courtesy of International Biophysics Corporation)
International Biophysics Corporation recently announced the publication of new data regarding the effectiveness of its AffloVest airway clearance technology in patients with cystic fibrosis. The paper, “Lung function improvement with AffloVest® HFCWO use: a clinician’s perspective on PFT score data from 12 patients with cystic fibrosis,” was published on the company’s website.
AffloVest is a battery operated, portable High Frequency Chest Wall Oscillation (HFCWO) therapy device for people with cystic fibrosis, bronchiectasis, and other respiratory diseases. Oscillating therapy works to clear mucus and pathogens from the lungs of cystic fibrosis (CF) patients, helping to lower their risk of infections and inflammation. The AffloVest, unlike other HFCWO devices, uses oscillating motors rather than air bladders to free secretions in patients’ lungs.
The paper reports data, collected by respiratory therapists Michelle W. Tackett and Vivian P. Henderson, from 12 patients who used the AffloVest for an average of 78 days. A total of 25 people were included in the study, but only 12, or 48%, showed “significant increase” in lung function after an airway clearance treatment regimen using the vest.
AffloVest was reported to lead to improvements in pulmonary function test (PFT) scores in these patients. Specifically, data showed a 15.22% improvement in average FVC (forced vital capacity), a 17.41% improvement in FEV1 (forced expiratory volume in 1 second), and an 11.21% improvement in FEF 25-75% (forced expiratory flow 25–75%).
The remaining 13 participants (52% of total) did not show significant increases in lung function, but also had no decline in function, the report said.
Importantly, “[p]atients with the most FEV1 score improvements appear to be those who had little or no compliance [previously] using their air bladders vests, and that the AffoVest’s technology and portability, coupled with ease of use, helped increase compliance,” the authors noted.
“This new data demonstrates once again the improved pulmonary outcomes seen with AffloVest use in patients with severe respiratory conditions,” said H. David Shockley, Jr., International Biophysics’ CEO, in a press release. “International Biophysics continues to focus on bringing innovative, disruptive medical devices and technologies to market that improve treatment therapies and patient outcomes. We are very happy to know that our technology is helping patients benefit from improvement in their lung function. We expect to see increased clinician adoption of AffloVest technology in their patient’s airway clearance therapy,”
According to the release, this report confirms results from an independent 2015 AffloVest study conducted by Michael Cooper, also a registered respiratory therapist, that found improved FEV1, FEF and FVC ratios of over 10% for all patients together.







