Experimental CF Therapy CTP-656 by Concert Pharma Begins New Round of Testing
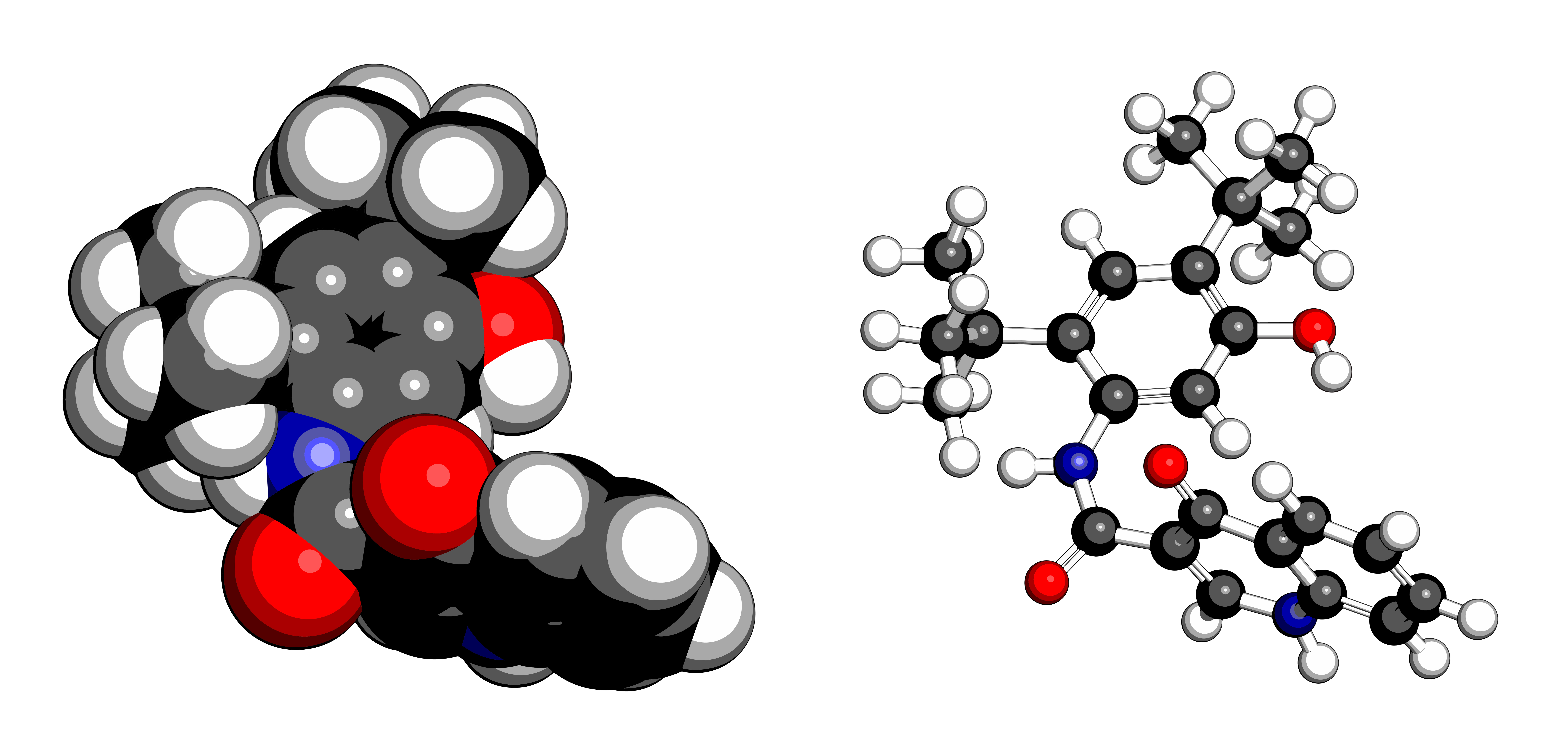
Concert Pharmaceuticals, Inc., a clinical stage pharmaceutical company that focuses on the development of new small molecule drugs through its DCE (deuterated chemical entity) Platform®, has announced the beginning of Phase 1 multiple ascending dose clinical trial with its cystic fibrosis treatment CTP-656 (deuterium-modified ivacaftor). The announcement follows recent news of early studies revealing that the investigational therapy outperformed Vertex’s Kalydeco.
Concert developed its DCE Platform to create new drug products with enhanced clinical safety, tolerability and efficacy. The platform usually uses approved or heavily studied compounds where deuterium chemistry is applied, resulting in a deuterated compound where hydrogen-carbon bonds are substituted by the more stable deuterium-carbon bonds. Drug metabolism usually involves the breaking of hydrogen-carbon bonds, this substitution can lead to more metabolic stability, less production of toxic by-products and increased half-life. These effects are highly dependent on chemical structure of the compounds and on the positions where the hydrogen is substituted for the deuterium.
CTP-656, developed as a monotherapy or for use with other CFTR (cystic fibrosis transmembrane conductance regulator) modulators, was developed through the DCE platform by modification of ivacaftor (Kalydeco®). Initially, Concert is developing CTP-656 as a monotherapy for minimal mutations of the CFTR gene and cystic fibrosis gating.
James Cassella, Ph.D., Chief Development Officer of Concert Pharmaceuticals, commented on the announcement in a press release,“CTP-656 has the potential to be an important new treatment, expanding therapeutic options for the cystic fibrosis community. We believe the development of CTP-656 as a once-daily dose could help address adherence issues documented with the current standard of care by offering a simplified treatment option.”
The Phase 1 study, divided in two parts, will enroll 38 healthy individuals to evaluate the pharmacokinetics, safety and tolerability of the drug. In the first part of the study, pharmacokinetic results from 150 mg of CTP-656 and 150 mg of Kalydeco will be compared. The second part will be an ascending dose study of the compound, starting at 75 mg, up until 300 mg daily for seven days compared to placebo administration. The results of the study are expected to be presented in the first half of 2016. Moreover, Concert plans the initiation of a Phase 2 study in CF patients in the second half of 2016.